The blood vasculature in the brain is a highly ramified, complex but well-organized vessel network. During development, the pathfinding of growing vessels is critical for the patterning of the brain vasculature. However, its underlying mechanism still remains elusive. A recent study published online in Neuron uncovers that Ca2+ activities mediated by mechanosensitive Piezo1 channels regulate the pathfinding of growing brain vessels in larval zebrafish. This work was performed by Dr. DU Jiulin’s research group.
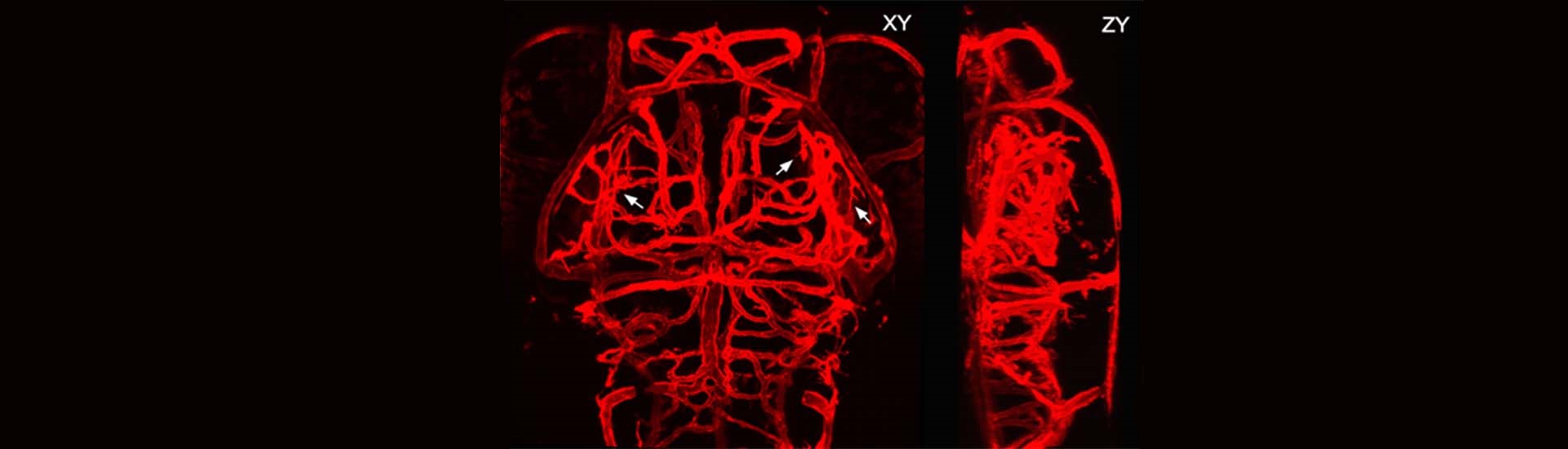
A recent study published in Nature Biotechnology reported a novel volumetric imaging method: confocal light field microscopy to image fast neural and vascular dynamics at high speed and deep in the brain. This work was performed by researchers in Dr. WANG Kai’s Lab and Dr. DU Jiulin’s lab.
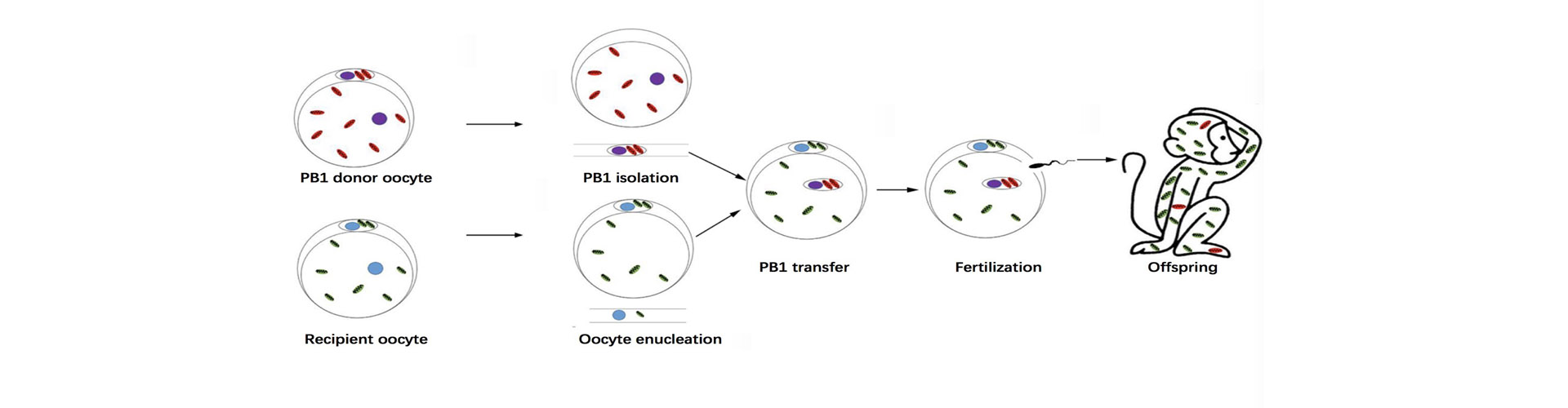
A recent study published in Cell Research reported the first monkey model for mitochondrial replacement by first polar body transfer. The study was conducted by a team led by SUN Qiang and LIU Zhen. This work is the first to demonstrate the effectiveness and safety of polar body replacement at the individual primate level, and has important reference significance for the treatment of mitochondrial genetic diseases and infertility caused by low ovarian reserve.
A recent study published in Journal of Cell Biology systematically analyzed the lineage progression in zebrafish retina and provided a proof-of-concept method to get specific neuron types through lineage-dependent reprogramming. This work was conducted by researchers from Dr. HE Jie’s Lab.
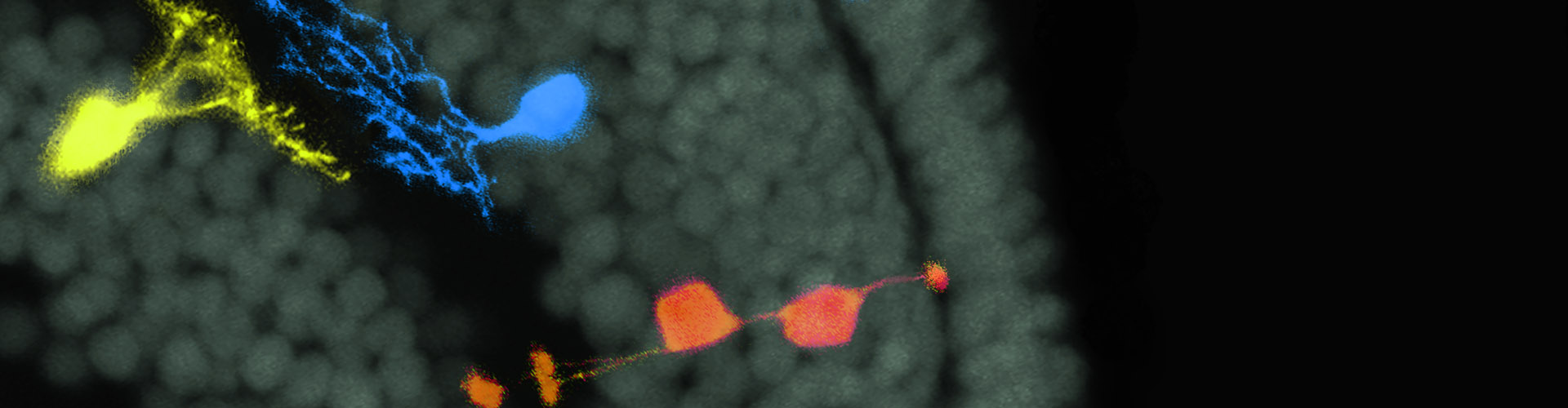
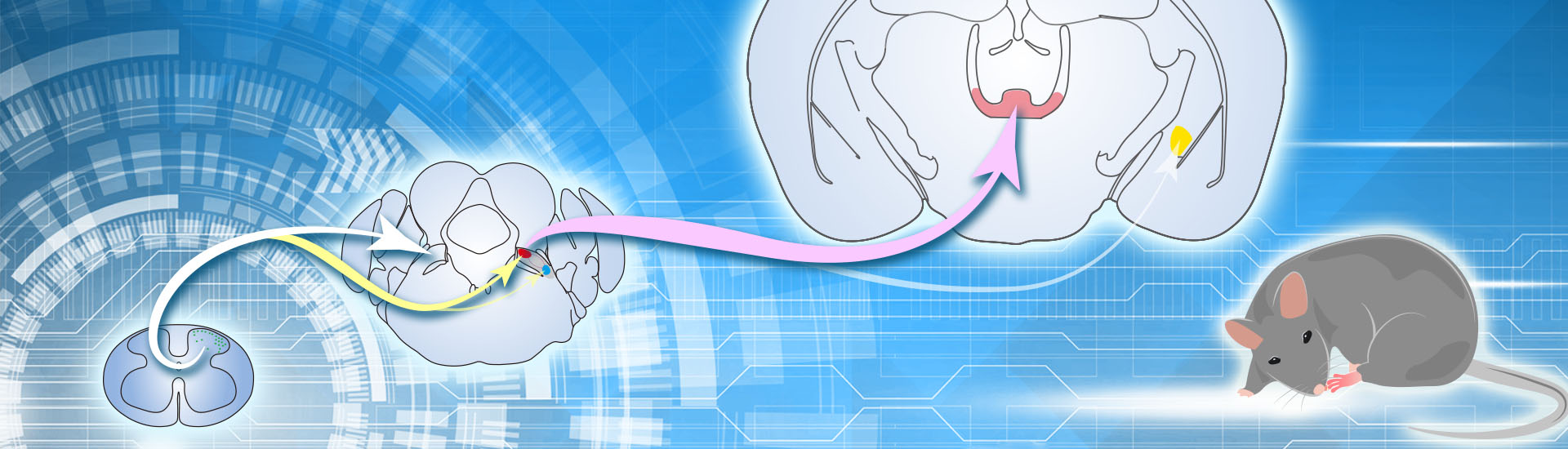
A recent study published in Neuron reveals the long-range circuit underlying nociceptive information processing. This work was performed by researchers in Dr. SUN Yangang’s Lab. The results demonstrate that the ipsilateral spino-parabrachial pathway directly relay pain signals from the spinal cord to the ILN but not the amygdala, providing crucial insight into the cellular and circuitry mechanism underlying nociceptive information processing.
A recent study published in eLife reveals that the secondary motor cortex contributes to adaptive action selection during flexible visual categorization. This work was performed by researchers in Dr. YAO Haishan’s Lab.

A recent study published in American Journal of Psychiatry reported a novel cross-species translational approach for diagnostic classification in human mental disorders. This work was performed by researchers in Dr. WANG Zheng’s Lab, in close collaboration with Dr. HE Ran’s group .

Two recent research articles revealed a considerable degree of plasticity of retinal function during the early stage of development. The research was completed by Dr. ZHANG Yifeng’s lab.The research results provide a basis for exploring how environmental stimuli change the retinal circuits, and help to explore the use of early sensory experience training for adjuvant treatment of some sensory system diseases.

A recent study published in eLife reveals the different neural mechanisms underlying opioid analgesia. This study was performed by researchers in Dr. SUN Yan-Gang’s Lab.Using a combination of genetic, pharmacological, and behavioral approaches, the authors reveal that analgesic effects of exogenous and endogenous opioids on inflammatory pain are mediated by mu opioid receptors (MORs) expressed in glutamatergic and GABAergic neurons, respectively.

A recent study published in Cell Reports demonstrates that retinoid X receptor α (Rxra) regulates docosahexaenoic acid (DHA)-dependent spinogenesis and functional synapse formation in vivo. This work was performed by researchers in Dr. YU Xiang’s Laboratory. Using a combination of gene expression manipulations (transgenic mice, viral injections and in utero electroporation), pharmacology, immunohistochemistry, RNA-Seq, fluorescence dye microinjection, neuronal morphology analyses, slice electrophysiology and behavioral tests, they identified an Rxra-dependent unesterified DHA signaling pathway required for functional synapse formation, providing insight into the mechanism through which DHA promotes brain development and cognitive function.
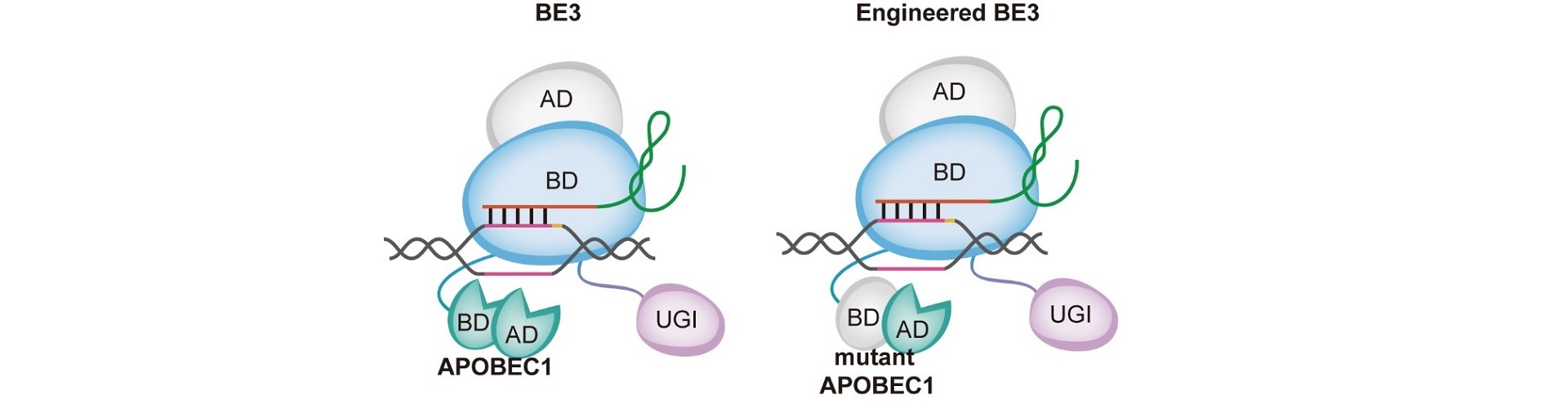
On May 18th, a research article named ”A rationally engineered cytosine base editor retains high on-target activity while reducing both DNA and RNA off-target effects” was published in Nature Methods, which was cooperatively accomplished by YANG Hui’s group, Li Yixue’s group and Zuo Erwei’s group.
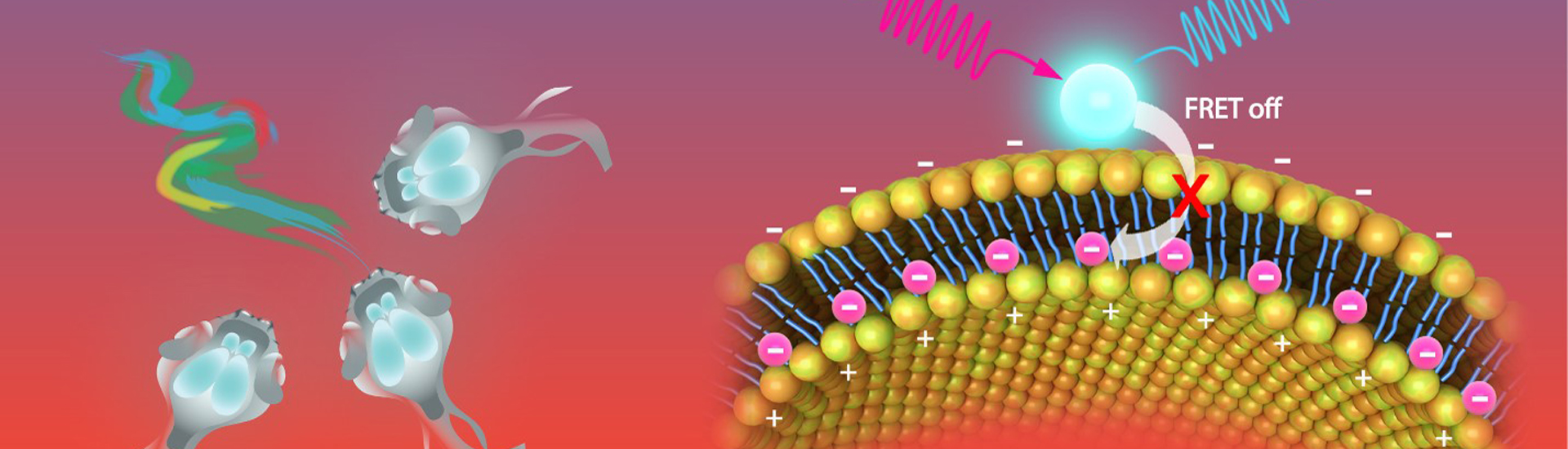
A recent study published in Science Advances reports a highly sensitive and selective potassium ion (K+) nanosensor that can be excited by near-infrared (NIR) light. This work was performed by researchers in Dr. DU Jiulin’s, Dr. XIONG Zhiqi’s Labs and Dr. BU Wenbo’s Labs.
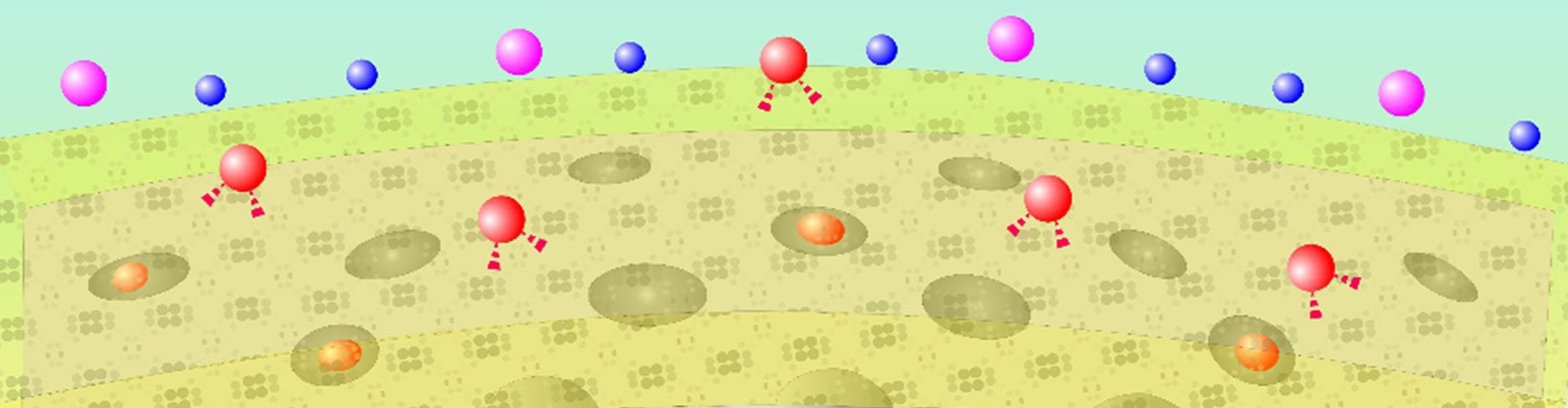
A recent study published in Journal of the American Chemical Society reports a near-infrared (NIR) voltage nanosensor that enables real-time imaging of neuronal activities in mice and zebrafish. This work was performed by researchers at Dr. DU Jiulin’s Lab.
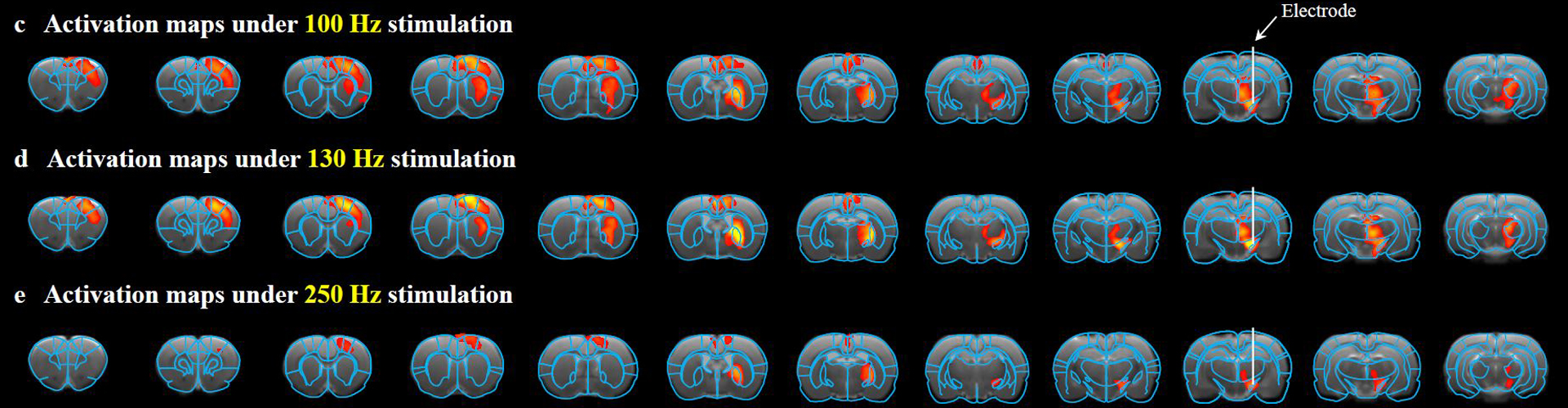
Recently, collaboration between Dr. DUAN Xiaojie’s group from Peking University and Dr. LIANG Zhifeng’s group from the Center for Excellence in Brain Science and Intelligence Technology, CAS, has led to a novel MRI compatible, graphene fiber DBS electrode. Using a Parkinsonian rat model, this novel electrode achieved full activation pattern mapping by simultaneous deep brain stimulation and fMRI, and revealed close relationship between fMRI activation and DBS therapeutic improvement.
A recent study published in Cell revealed that glia-to-neuron conversion alleviates symptoms of two top-ranked neurological diseases in mice. This work was performed by researchers in Dr. YANG Hui’s Lab. This study showed that knockdown of a single gene Ptbp1 using a recently developed RNA-targeting CRISPR system CasRx, resulted in the conversion of Müller glia (MG) into retinal ganglion cells (RGCs) in mature retinas, leading to the restoration of visual responses in a mouse model with permanent vision impairment. Using a similar approach, it demonstrated that knockdown of Ptbp1 in the striatum locally induced neurons expressing dopaminergic markers with a very high efficiency and alleviated motor dysfunctions in a mouse model of Parkinson’s disease (PD). Together, this study provides a new approach for treating a variety of disorders due to neuronal loss.
