Time:2020-08-11
A recent study published in Nature Biotechnology reported a novel volumetric imaging method: confocal light field microscopy to image fast neural and vascular dynamics at high speed and deep in the brain. This work was performed by researchers in Dr. WANG Kai’s Lab and Dr. DU Jiulin’s lab at the Institute of Neuroscience, State Key Laboratory of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences.
Understanding the multiscale integration of information and the emergence of behaviors from the global dynamics of large neuronal populations is a fundamental problem in current neuroscience and requires fast three-dimensional imaging in large brains. Traditional tools developed for in-vivo imaging in the brain, such as confocal microscopy and two-photon scanning microscopy, are based on point scanning scheme and too slow to study rapid volumetric dynamics. Therefore, considerable efforts have been devoted to developing faster imaging strategies recently. Among them, Light Field Microscopy (LFM) is of particular interest due to its instantaneous volumetric imaging capability, which captures the information from the whole 3D volume all together on the image sensor in a single camera exposure.
Conventional LFM has been previously introduced to image brains, however, they suffer from two major problems that prevent them from wide spread applications, including reconstruction artifacts and lack of optical sectioning capability. In 2017, WANG’s group developed a new type of LFM, termed XLFM, to address the first issue and demonstrated its successful application on whole brain functional imaging of neural activities in freely swimming larval zebrafish. However, the second issue was unresolved and limited its wider applications on imaging larger brains, such as mouse brains.
Introducing optical sectioning capability in LFM is of key importance because it prevents out-of-focusing background from interfering with in-focusing signal and plays vital role for thick tissue imaging. Directly adapting the concept of confocal detection that helps confocal scanning microscope to gain 3D imaging capability seems to be straight forward. However, it turned out impossible due to the very distinct instantaneous volumetric imaging manner of LFM from conventional plane-by-plane imaging scheme. To meet this challenge, WANG’s lab came up with a novel idea to generalize the concept of confocal detection and made it compatible with LFM without compromising its imaging speed, which was termed Confocal LFM.
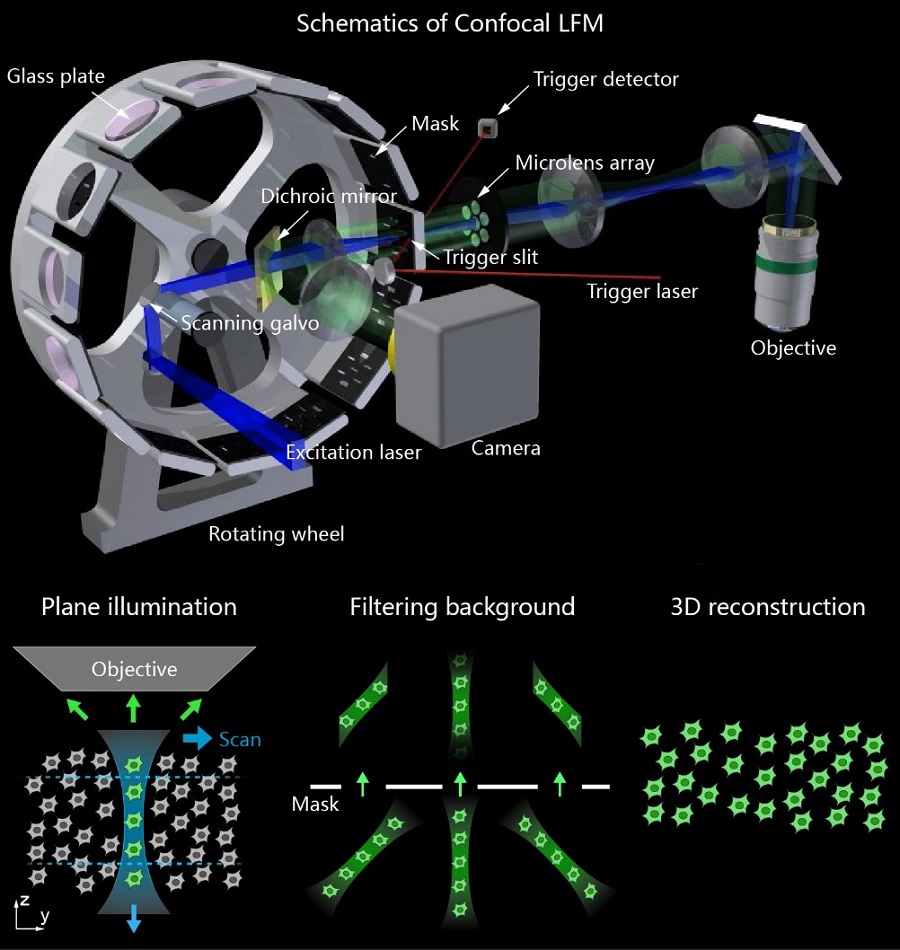
Figure 1 Schematics of Confocal LFM. (Upper) Overview of the optical system. (Lower) Vertical light sheet scans in the sample plane and images formed by micro-lens array are spatially filtered by the mask to get in-focus volume after 3D reconstruction.(Image by CEBSIT)
Due to the elimination of interfering background, Confocal LFM significantly improved imaging resolution and calcium signal detection sensitivity in functional imaging of neuronal activities over the whole zebrafish brain. It was also integrated with a fast 3D tracking system to capture behavioral correlated neural activations in freely swimming larval zebrafish. For example, single-neuron activities during larval zebrafish’s prey capture can be reliably recorded.
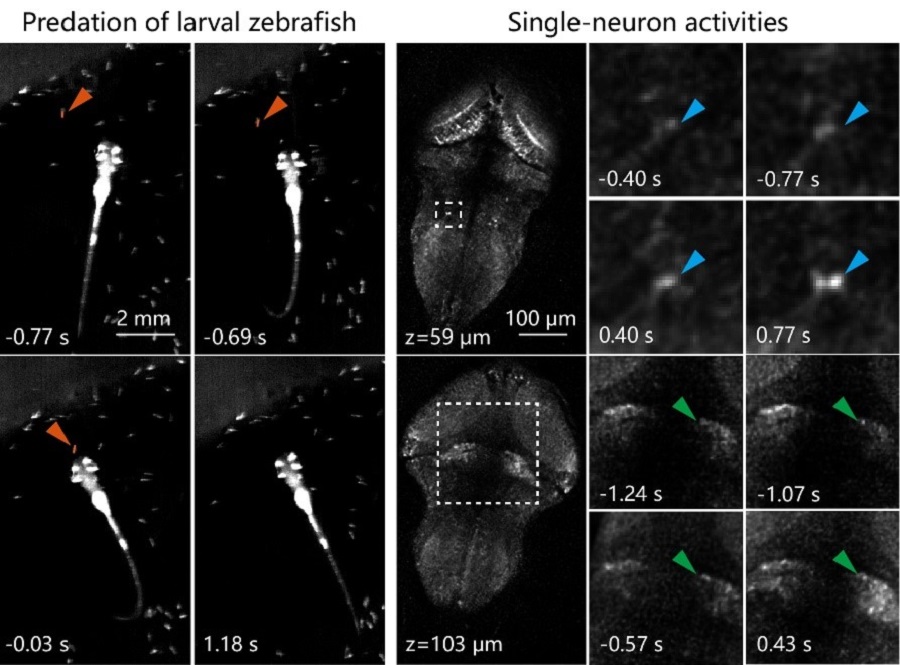
Figure 2 (Left) An example of larval zebrafish’s prey capture. Time was aligned to prey ingestion (0s). (Right) Zoomed-in views of single-neuron activities in several brain regions during prey capture in left. (Image by CEBSIT)
Authors further applied Confocal LFM on imaging calcium transients and circulating blood cells in awake mouse brain. They demonstrated high speed calcium imaging over a volume of 800 x 800 x 150 μm3 and up to ~400 μm deep into the mouse cortex. It’s highly parallelized and light efficient imaging manners enabled continuous recording for more than 100,000 volumes without significant photobleaching. They also showed imaging of populations of circulate blood cells in a complicated 3D vascular network consisting of thousands of blood vessel branches at 70 volumes/s and up to 600 μm deep into awake mouse cortex, which made it possible to track and quantify the flow dynamics of blood cells accurately and represented more than 100 times increase in throughput comparing to conventional imaging techniques. The flexibility, increased resolution and sensitivity of Confocal LFM demonstrated in this work showed great promise for its wide applications on capturing and studying extremely fast volumetric dynamics deep in the brain, or other types of thick tissues in general.

Figure 3 (Left) Vascular network in cortex of mouse captured by Confocal LFM. Six volumes at different depths are stitched together as one volume of more than 600 μm in z. (Middle) Projection of the vascular network in the depth range between 100 and 250 μm. Colors represent average velocities of blood cells in different branches. (Right) Counting of blood cells traveling through different branches of a blood vessel. The analyzed blood vessel is indicated by the arrow in middle. (Image by CEBSIT)
This work entitled “Imaging volumetric dynamics at high speed in mouse and zebrafish brain with confocal light field microscopy” was published online in Nature Biotechnology on August 10, 2020. ZHANG Zhenkun, BAI Lu and CONG Lin are the first authors with equal contribution. This work was supported by the Chinese Academy of Sciences, NSFC, Ministry of Science and Technology of the People′s Republic of China, Shanghai Municipal Government.
https://www.nature.com/articles/s41587-020-0628-7
AUTHOR CONTACT:
WANG Kai
Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, Shanghai, China.
E-mail: wangkai@ion.ac.cn
 附件下载:
附件下载: