Time:2020-08-22
The blood vasculature in the brain is a highly ramified, complex but well-organized vessel network. During development, the pathfinding of growing vessels is critical for the patterning of the brain vasculature. However, its underlying mechanism still remains elusive.
A research team led by Dr. DU Jiulin of the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of Chinese Academy of Sciences, has revealed that Ca2+ activities mediated by mechanosensitive Piezo1 channels regulate the pathfinding of growing brain vessels in larval zebrafish.
The formation of the brain vasculature is initiated by vessel invasion from the surrounding perineural vascular plexus around the ventral neural tube during early development, and then the brain vasculature is expanded by the continuous emergence and growth of newborn vessels from preexisting ones, a process known as angiogenesis.
Endothelial tip cells (ETCs) located at the forefront of growing vessels usually extend a few motile primary branches and many dynamical fine filopodia, navigate through tissue microenvironments, and steer angiogenic sprouts to their appropriate targets. This process of ETC pathfinding is critical for the proper patterning of the vasculature in the brain as well as in all other organs, but its underlying mechanism still remains largely unknown.
To investigate the cellular and molecular mechanism underlying ETC pathfinding, Dr. DU's group first monitored the entire process of ETC pathfinding during brain vascular development through in vivo long-term time-lapse simultaneous imaging of both the morphological dynamics and Ca2+ activity of ETCs in larval zebrafish.
They found that before reaching target vessels, ETCs frequently extended and retracted subcellular primary branches, leading to continuous changes in the direction of ETC migration and vessel growth.
Interestingly, branches of ETCs exhibited spontaneous local Ca2+ transients at different frequencies during pathfinding, with high- and low-frequency local Ca2+ transients associated with branch retraction and extension, respectively.
Then the researchers examined the causal relationship between local Ca2+ transients and fate determination of ETC branches via local manipulation of Ca2+ concentration at ETC branches, and found that high- and low-frequency Ca2+ transients were necessary and sufficient for the retraction and extension of ETC branches, respectively.
Furthermore, they investigated the origin of local Ca2+ activities of ETCs, and discovered that mechanosensitive Piezo1 cationic channels were preferentially expressed on ETC branches and activated by tissue stiffness-associated mechanical force. They mediated local Ca2+ activities of ETC branches, thus regulating the retraction and extension of ETC branches.
Mutating piezo1 largely diminished local Ca2+ transients of ETC branches, impaired the pathfinding of ETCs, and therefore disrupted the patterning of the brain vasculature.
Finally, the researchers showed that the protease calpain and nitric oxide synthase mediated the effects of Piezo1-mediated Ca2+ activities on ETC branch retraction or extension, respectively.
This study reveals that Piezo1 and downstream Ca2+ signaling act as molecular bases for ETC pathfinding and highlights a novel function of Piezo1 and Ca2+ in vascular development.
This work entitled “Piezo1-Mediated Ca2+ Activities Regulate Brain Vascular Pathfinding during Development” was published online in Neuron on August 21, 2020. It was performed mainly by Dr. LIU Tingting, with help from Dr. DU Xufei, Dr. ZHANG Baibing, ZI Huaxing, YAN Yong, Dr. YIN Jiang-an, Dr. HOU Han, Dr. GU Shanye, and Dr. CHEN Qi, under supervision of Dr. DU Jiulin at the Institute of Neuroscience, Chinese Academy of Sciences. It was financially supported by Shanghai Municipality and Chinese Academy of Sciences.
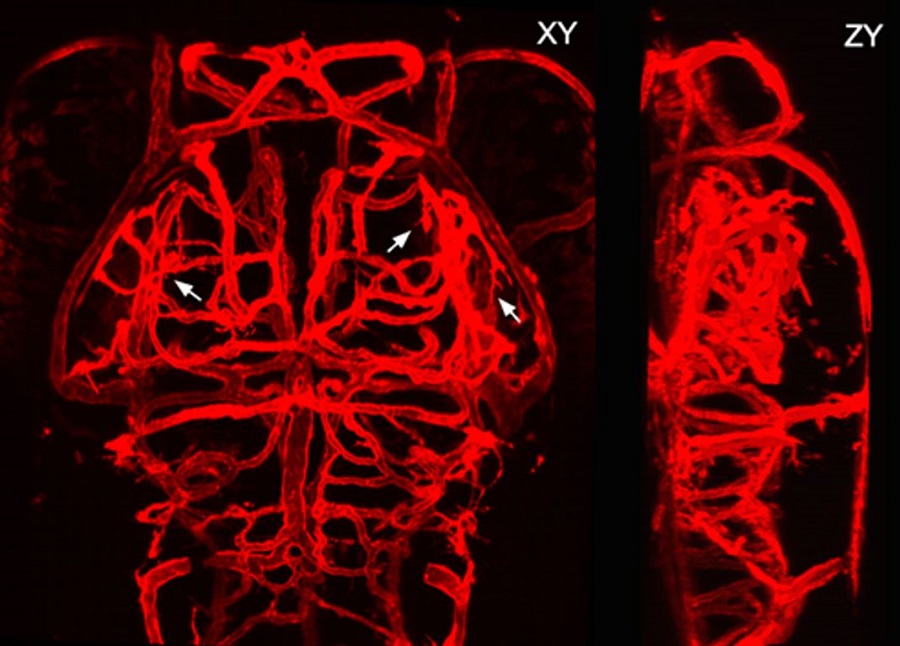
Fig.1 Z-axis and X-axis projected confocal images of the brain vasculature of a larval zebrafish (Image by CEBSIT)
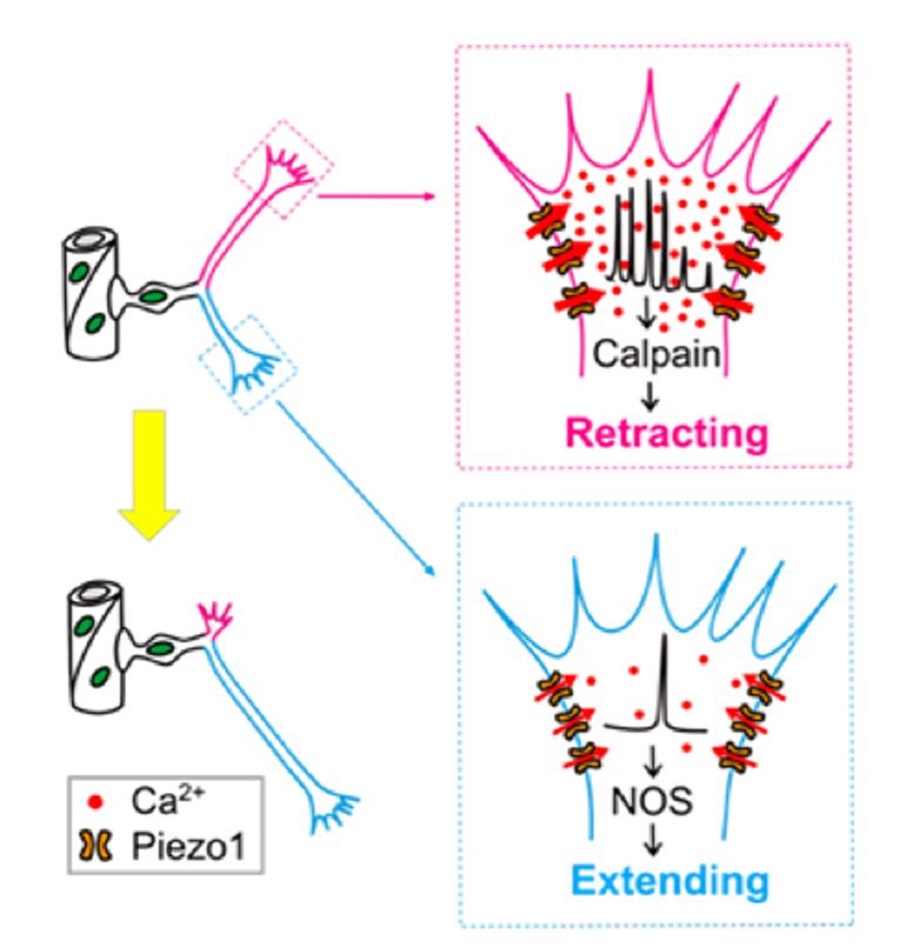
Fig.2 Pathfinding selection mechanism of endothelial tip cells (Image by CEBSIT)
AUTHOR CONTACT:
DU Jiulin
Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.
E-mail: forestdu@ion.ac.cn
 附件下载:
附件下载: