Time:2022-03-23
Recently, researchers successfully constructed a crab-eating monkey model with early-onset epileptic encephalopathy through single-base editing technology. The model reproduces the typical clinical features of human patients in aspects of genotype, neuron, brain activity (EEG), behavior, and drug interventions. The establishment of this model also provides new tools for subsequent disease mechanism research, intervention exploration, and drug testing. This research was mainly completed by the SUN Qiang’s Group and the LIU Zhen’s Group at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligent Technology of the Chinese Academy of Sciences, Shanghai Research Center for Brain Science and Brain-like Research and the Key Laboratory of Primate Neurobiology of the Chinese Academy of Sciences, and the Huang Xingxu Group of ShanghaiTech University.
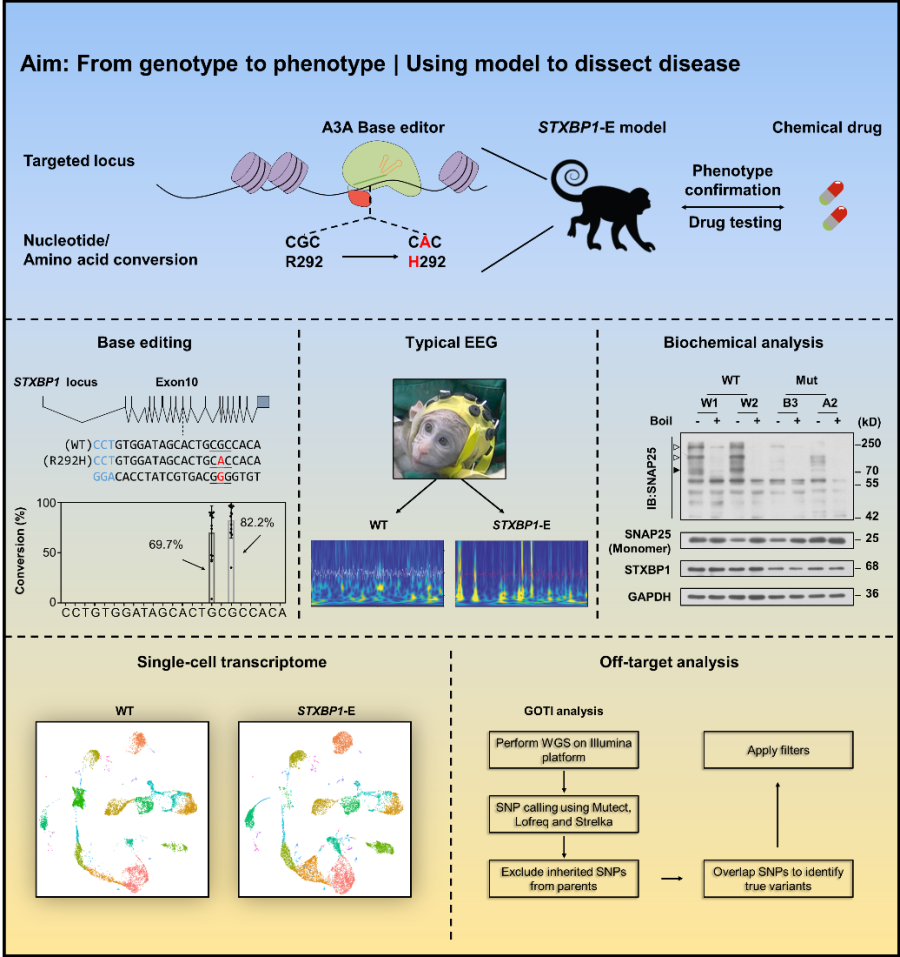
Presynaptic synaptic fusion protein binding protein 1 (STXBP1) is essential for neurotransmitter release. Heterozygous mutations in the STXBP1 gene can lead to STXBP1 encephalopathy (STXBP-E) in early childhood with the main symptoms of intellectual disability and epilepsy. Despite the advances in studies of mouse models, they do not mimic severe seizures and typical inhibitory EEG patterns in STXBP1-E patients. Considering that non-human primate laboratory animals are evolutionarily closer to humans, this team generate a cynomolgus model with a STXBP1 mutation.
The study first recapitulate the hotspot mutation R292H on the STXBP1 gene using cytosine monobase editor (A3A). Authors performed embryo microinjection and intracytoplasmic sperm injection technology, and then transferred the edited embryos to the uterus of surrogate monkeys, and obtained 2 birth individuals and 3 miscarriage monkeys. The results of genotype identification and chimeric rate analysis showed that the genotype of birth monkey was close to heterozygous mutation, while that of the abortion monkey was close to homozygous mutation These results were consistent with the reports of homozygous and lethal mutations of the STXBP1 gene.
Phenotypic analysis and behavioral observations showed that typical epilepsy symptoms such as focal epilepsy and unilateral clonus were observed in both birth monkeys after excluding myoclonus (transient, convulsive, shock-like involuntary movements). Further EEG studies found that the EEG of STXBP1 mutant monkeys had a typical epileptic discharge and burst suppression pattern (EEG). Burst-suppression EEG is widely recognized as an essential feature of deep brain inactivation, common in deep anesthesia, hypothermia, coma, and early-onset infantile encephalopathy. This kind of EEG pattern is often associated with a poor prognosis. In animal models of epilepsy, these typical burst-suppression EEG patterns were the first to be discovered and reported. At the same time, we also found that the low frequency oscillation in the EEG traces of STXBP1 mutant monkeys was significantly higher than that of wild monkeys. This phenomenon was also seen in reports of refractory epilepsy in infants, and was known as infantile spasms biomarker. In this report, the researchers then tracked levetiracetam drug interventions and EEG levels in disease monkey. Unfortunately, after administration, epilepsy symptoms and EEG patterns were not alleviated significantly.
Discharge (presumed as epileptic foci) is concentrated in the prefrontal lobe and occasionally metastasizes to the occipital region, which also reflects the poor efficacy of current drugs for the treatment of refractory epilepsy.
The researchers performed biochemical analysis and found that the R292H mutation can block the binding of the STXBP1 protein to the Syntaxin1 protein. STXBP1 mutant monkey brain tissue showed significant reductions in the SNARE complex, STXBP1 protein, and Syntaxin1 protein. The above results indicate that the function of neurotransmitter release in monkeys with STXBP1 mutation is affected. To further exploit the STXBP1 mutant monkey model, the researchers performed a single-cell nuclear transcriptome experiment after sampling the prefrontal cortex of an STXBP1 mutant monkey. The results showed that the most obvious change was a significant decrease, or even disappeared, in the number and proportion of ExN7 neurons in mutant monkeys. Gene ontology analysis showed that the development of such neurons is affected, suggesting a possible role for specific neurons in the development of disease. The study also carefully evaluated the potential off-target effects of the base editor during model generation, and the results showed that despite the presence of obvious deaminase-based random off-target effects, the off-target sites were not associated with the simulated disease phenotype.
The study obtained a non-human primate model of epileptic encephalopathy for the first time using base editing technology, and proved that the model can better recapitulate human epileptic encephalopathy, demonstrating the advantages and prospects of non-human primate models in the study of neurologically related diseases.
This research entitled "Base-edited Cynomolgus Monkeys mimic core symptoms of STXBP1 encephalopathy" was published online in the journal of Molecular Therapyon March 23, 2022. Dr. LU Zongyang, Dr. HE Siting, Graduate Student ZHUANG Ling, and Dr. JIANG Jian are the co-first authors of the paper. Dr. SUN Qiang and Dr. LIU Zhen of the Center for Excellence in Brain Science and Intelligent Technology of the Chinese Academy of Sciences and Dr. HUANG Xingxu of ShanghaiTech University are co-corresponding authors of the paper. This project has also received suggestions and guidance from Dr. XIONG Zhiqi and Dr. Zhang Hungchun of the Center for Brain Intelligence Excellence, and Dr. Cao Peng of Beijing Institute of Life Sciences. The paper editing was guided by Dr. POO Muming, Scientific director of the Center for Brain Intelligence Excellence. This work has been supported by the Chinese Academy of Sciences, Shanghai Municipality, the Ministry of Science and Technology and the Fund Committee.
Keywords: Base-edited Cynomolgus Monkeys;STXBP1 encephalopathy
AUTHOR CONTACT:
SUN Qiang
Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.
E-mail:qsun@ion.ac.cn
 附件下载:
附件下载: