Time:2020-07-08
A recent study published in Neuron reveals the long-range circuit underlying nociceptive information processing. This work was performed by researchers in Dr. SUN Yangang’s Lab at the Institute of Neuroscience, State Key Laboratory of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences.
Pain sensation is critical for the animals to sense the changing environment for potential danger, and is thus important for the survival of the animals. However, chronic pain still remains a major challenge in clinic, affecting the quality of life for more than 15% of the population.
Mechanistic study of the nociceptive information processing will help to identify new therapeutic target for treatment of chronic pain. Major progress has been made in understanding the cellular and molecular mechanism for nociceptive information processing at the spinal level. However, it still remains elusive how nociceptive signals are transmitted from the spinal cord to the brain.
Previous studies showed that spinal projection neurons send projections to multiple brain areas, including the thalamus, periaqueductal gray and the parabrachial nucleus (PBN). Using the viral tracing approaches, the authors demonstrated that the spinal cord connects with PBN in a bilateral manner, which is different from spinal projection targeting at other brain areas with a dominant contralateral pattern.
They further examined the functional role of the ipsi- and contralateral spino-parabrachial pathways in processing nociceptive information. They found that inhibition of the ipsilateral, but not the contralateral, spinoparabrachial pathway suppressed the formalin-evoked pain-related licking behaviors. Consistently, activation of the ipsilateral rather than the ipsilateral spinoparabrachial evoked pain-related licking behaviors.
Further, they identified Tacr1-expressing neurons as the major neuronal subtype in PBN that receives direct spinal input. These Tacr1-expressing neurons were selectively activated by noxious mechanical and thermal stimulation, and were critical for processing nociceptive information.
It has been long thought that PBN neurons that receive projections from the spinal cord directly relay pain relayed signals from the spinal cord to the central nucleus of amygdala. The authors showed that PBN neurons receiving spinal input form functional monosynaptic excitatory connections with neurons in the intralaminar thalamic nuclei (ILN) but not the amygdala.
Together, these results demonstrate that the ipsilateral spino-parabrachial pathway directly relay pain signals from the spinal cord to the ILN but not the amygdala, providing crucial insight into the cellular and circuitry mechanism underlying nociceptive information processing.
This work entitled “The parabrachial nucleus directly channels spinal nociceptive signals to the intralaminar thalamic nuclei, but not the amygdala” was published online in Neuron on July 9, 2020. This work was completed by Dr. DENG Juan, under the supervision of Dr. SUN Yangang, with help from ZHOU Hua, LIN Junkai, SHEN Zixuan, CHEN Wenzhen, WANG Linhan, LI Qing, MU Di, WEI Yichao and Dr. XU Xiaohong. This work was supported by the National Natural Science Foundation of China, Shanghai Municipal Government, and Chinese Academy of Sciences.
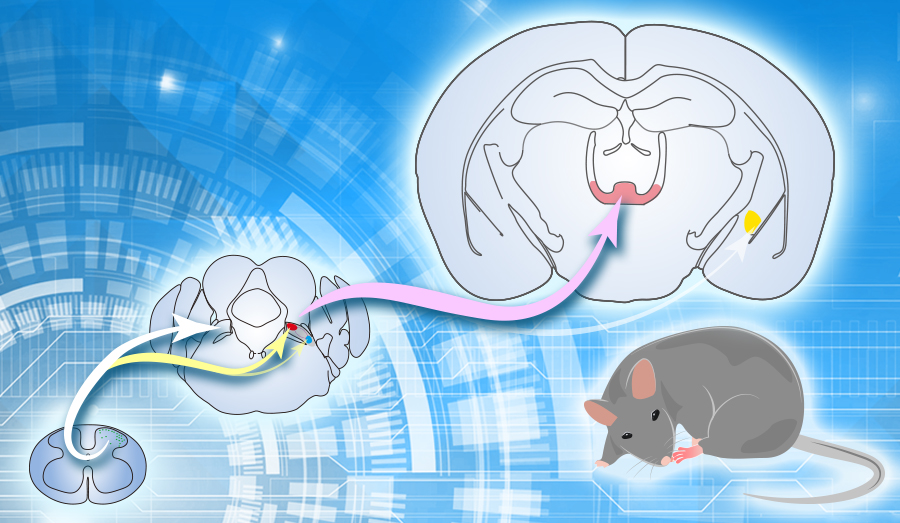
Figure legend: Ipsilateral spino-parabrachial pathway directly relay pain signals from the spinal cord to the ILN, but not the amygdala. Projection neurons in the spinal cord send projections to both sides of parabrachial nucleus (Contralateral: white line; ipsilateral: yellow line). Most of PBN neurons that receive projections from the spinal cord (PBNSC) are located in superior lateral PBN (Red), which send density projections to the intralaminar thalamic nuclei (ILN, Pink line) and few projections to amygdala (Blue thin line). Few PBNSC neurons are located in the external lateral PBN (Blue). (Image by CEBSIT)
AUTHOR CONTACT:
SUN Yangang
Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, Shanghai, China.
E-mail: yangang.sun@ion.ac.cn
 附件下载:
附件下载: