Time:2020-04-14
A recent study published in Journal of the American Chemical Society reports a near-infrared (NIR) voltage nanosensor that enables real-time imaging of neuronal activities in mice and zebrafish. This work was performed by researchers at Dr. DU Jiulin’s Lab at the Center for Excellence in Brain Science and Intelligence Technology, Institute of Neuroscience and State Key Laboratory of Neuroscience, and at Dr. SHI Jianlin’s and Dr. BU Wenbo’s Labs at State Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences.
Optical sensors of neuronal activity are extensively pursued as they expand our recording scope and understanding depth. Typically, the information processing in the brain is associated with changes in the membrane potential. Direct monitoring of membrane potential dynamics in the central nervous system is still considered as the most reliable method to uncover operational principles of the brain. Until now, most existing sensors are only excited by high-intensity visible light with a wavelength typically < 600 nm. However, such visible light suffers from distortion and scattering in deep tissues, preventing long-term imaging due to photo-bleaching. It is well-known that biological tissues have an “optical transparency window” in the NIR range of 750 nm - 1000 nm. Consequently, the development of NIR-excited voltage indicators is highly desirable.
Lanthanide-doped upconversion nanoparticles (UCNPs) are a promising new generation of imaging agents for bio-imaging. Upconversion luminescence is an anti-Stokes process whereby low-energy photons are converted to higher-energy ones. In this study, the researchers developed a voltage nanosensor consisting of two moieties: NIR-excited UCNPs anchored at the outer surface of cellular membrane and voltage-sensitive dipicrylamine (DPA) embedded within the cellular membrane. At resting potential, the negatively charged DPA molecules are more abundantly distributed near the outer surface of the cellular membrane. Therefore, the distance between DPA and UCNPs is relatively short, leading to a largely quenched UCNP luminescence because of the high FRET efficiency. In contrast, upon membrane depolarization, the DPA translocates to the inner side of the cellular membrane. The thickness of cell membrane is typically 7.5 nm - 10 nm. As a result, the FRET between UCNPs and DPA will no longer occur under this circumstance, which significantly brighten the UCNP luminescence. Therefore, based on the fluctuation of FRET efficiency between the UCNP and the DPA, the nanosensor luminescence can report the membrane potential.
To demonstrate the wide application of this new voltage nanosensor, the researchers used it in detecting neural activities in zebrafish and mice in vivo. Dynamic fluctuations in neuronal membrane potential always happen in the brain. However, this information is always missed by the previously developed fluorescent protein-based sensors because they not only suffer from the drawbacks of low signal-to-noise ratio but also rely on the repeated average to discriminate the evoked responses. In addition, recording duration of those sensors is relatively short due to the serious photobleaching. As a result, application has been limited. By using the newly developed voltage nanosensor, the researchers examined the pallial olfactory response in the larval zebrafish. Upon NIR irradiation, every single application of food extract resulted in an obvious increase in luminescence intensity of neurons. Such a phenomenon kept stable upon the repeated stimulations. In sharp contrast, no response from the food-responsive neurons could be observed when the control solution without food flavor was applied. These results clearly indicate that the responses of the nanosensor originate from their olfactory rather than mechanical stimulation. Thanks to the negligible photobleaching of the developed nanosensor, the recording duration can last for as long as 30 min.
Subthreshold oscillations in membrane potential of neurons in vivo reflect animal brain states. For example, slow wave oscillation is characteristic of deep sleep and deep anesthesia. Upon awakening, this low-frequency oscillation faded and even diminished, which was subsequently replaced by high-frequency oscillation. It is quite difficult for conventional calcium imaging to report such oscillations. By taking advantage of the developed voltage nanosensors, researchers successfully observed the changes of subthreshold oscillation before and after anesthesia. Under pentobarbiturate anesthesia, the nanosensors in the somatosensory cortex of mouse displayed luminescence oscillation at < 1 Hz, indicating slow wave oscillation in the brain. After tail pinch for increasing the mouse vigilance, slow wave oscillation reflected by the voltage nanosensors gradually diminished and recovered after 10 min. All these results clearly indicate that the luminescence intensity of the voltage nanosensor can reveal the change of subthreshold oscillations in the brain.
Taken together, this work successfully demonstrates the feasibility and reliability of using the developed voltage nanosensors to monitor neuronal activities in the brain in vivo.
This work entitled “Near-infrared voltage nanosensors enable real-time imaging of neuronal activities in mice and zebrafish" was published online in Journal of the American Chemical Society on April 8, 2020. LIU Jianan, ZHANG Rongwei, and SHANG Chunfeng contribute equally as the first authors. Dr. DU Jiulin, Dr. SHI Jianlin, and Dr. BU Wenbo are corresponding authors. It was financially supported by Chinese Academy of Sciences, Science and Technology Commission of Shanghai Municipality, National Natural Science Foundation of China, the Institute for Basic Science of Korea, and Guangdong Innovation Platform of Translational Research for Cerebrovascular Diseases.
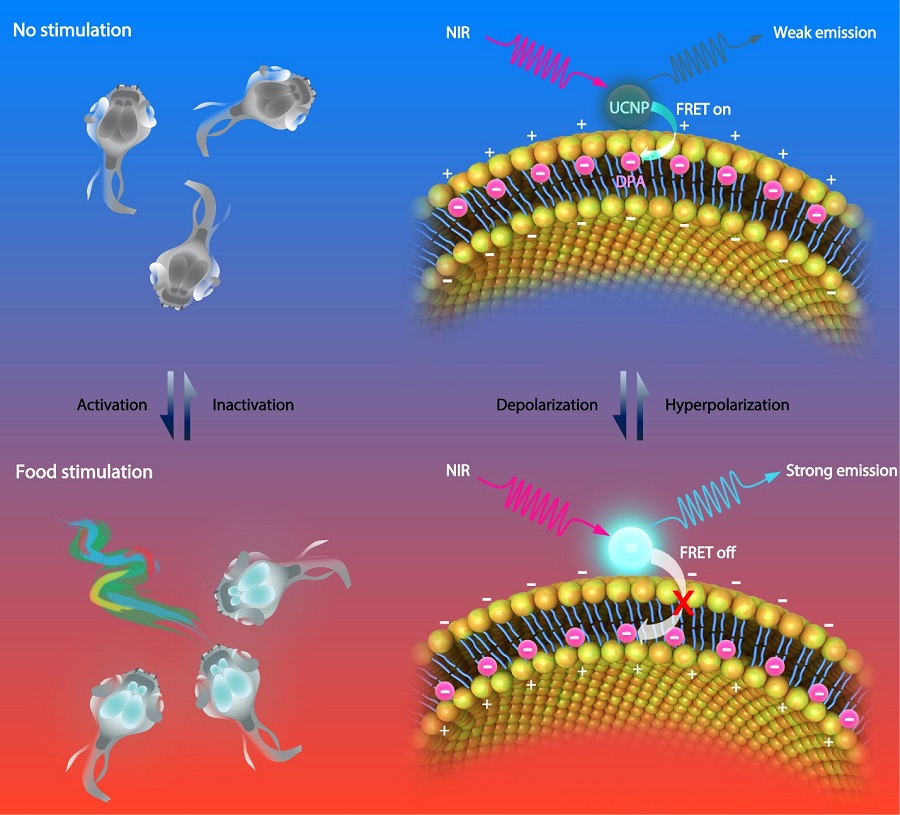
Figure legend. Schematic illustration showing the basic mechanism of the voltage nanosensor. UCNPs upconvert NIR to UV-Vis luminescence emissions of different intensities under varied membrane potentials, due to the distance-dependent changes in the FRET efficiency between the UCNP and DPA. Under the hyperpolarized state, DPA molecules prefer to locate close to the outer leaflet of the plasma membrane, leading to increased efficiency of FRET and decreased luminescence intensity of UCNPs (Top). Under the depolarization state, the translocation of DPA to the inner leaflet of the plasma membrane decreases the efficiency of FRET due to the increased distance between the two moieties, resulting in an increased UCNP luminescence (Bottom). (Image by CEBSIT)
Key words: voltage nanosensor, upconversion nanoparticles, near-infrared light, zebrafish, subthreshold oscillation.
AUTHOR CONTACT:
DU Jiulin
Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.
E-mail: forestdu@ion.ac.cn
 附件下载:
附件下载: