Time:2021-05-18
A recent study published in Cell Reports demonstrated that FGF13 gene acts as an intrinsic regulator in the maintenance of the self-renewal and proliferation of NSCs in the hippocampus during the postnatal development. This work was jointly performed by Dr. ZHOU Jiawei’s Lab at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, and Dr. HU Gang’s group at the Nanjing Medical University.
The development and plasticity of the hippocampal dentate gyrus (DG) are crucial for behaviors, such as learning and memory. In the mouse brain, most of granule cells in the DG are generated during early postnatal stages. The neurogenic capacity of NSCs in the DG drops sharply during the first two postnatal weeks and then gradually declines with age in adulthood. However, the molecular mechanism underlying this process remains elusive. FGF13 belongs to a subgroup of non-secretory fibroblast growth factor (FGFs) family. Human genetic studies have revealed that Fgf13 is a candidate gene of several X-linked brain disorders including mental retardation and epilepsy with febrile seizures plus, indicating the important roles of FGF13 in brain development. A previous study by Dr. Zhang Xu in the same institute has shown that FGF13 takes part in regulating polarity/migration of immature neurons in the forebrain during embryonic stage.
The two research teams have found that expression of isoform A of FGF13 was decreased in NSCs with age-related decline of DG neurogenesis. Deficiency of FGF13 in NSCs caused remarkabe impairment of postnatal neurogenesis in the DG, and hippocampus-related learning and memory behaviors of mice. Mechanistically, FGF13A isoform is essential for maintaining the capacity of self-renewal of NSCs by modifying chromatin structure and regulating the genes involved in neuron differentiation. The yeast two-hybrid assay suggests that FGF13A interacted with ARID1B, a crucial component in the Brahma-associated factor (BAF) chromatin remodeling complex thereby regulating the development of NSCs.
The results of assay for transposase accessible chromatin with high throughput sequencing (ATAC-seq) suggests that FGF13 was required for the maintenance of close chromatin state of neuronal differentiation-related genes in order to maintain the self-renewal and proliferative state of NSCs. Thus, the normal development of hippocampus requires proper spatiotemporal coordination of FGF13 isoform expression (Figure 1). This study indicates that FGF13 is important for the prediction and intervention of mental disorders associated with postnatal development and aging.
This paper entitled “Nuclear isoform of FGF13 regulates postnatal neurogenesis in hippocampus through epigenomic mechanism” was published online in Cell Reports on May 18, 2021, with YANG Qiaoqiao being the first author of this paper. This work was supported by the CAS, MOST and NSFC.
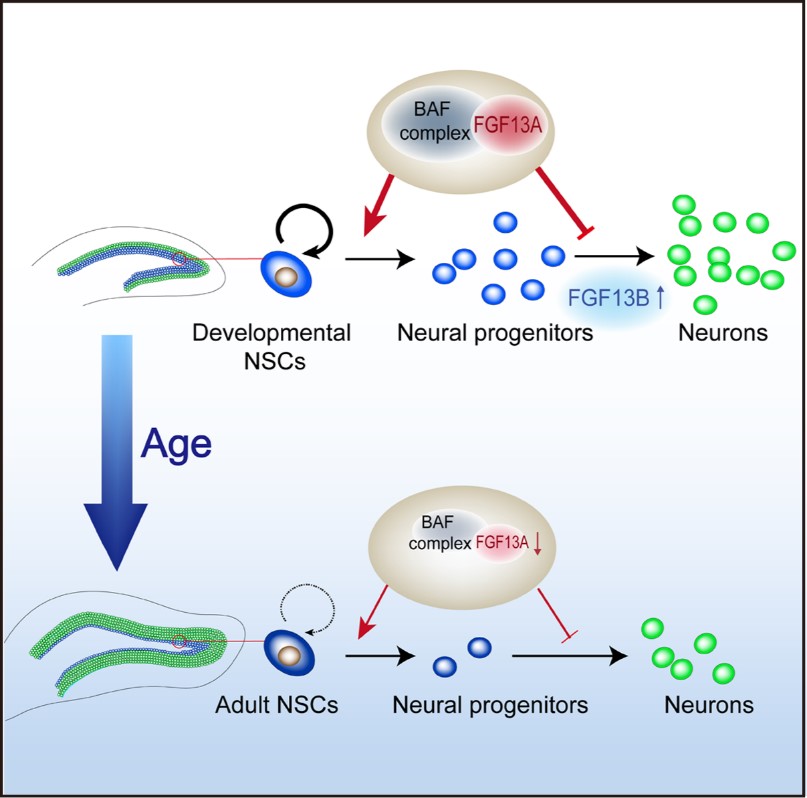
Figure legend: FGF13 takes part in the regulation of hippocampal development of mice. The expression levels of FGF13A are downregulated with the decline of neurogenesis ability of NSCs in hippocampus DG during the development and ageing of mice, while the FGF13B is upregulated during the differentiation of NSCs. The nucleus localized isoforms FGF13A maintains NSCs in the DG by suppressing neuronal differentiation, possibly through regulating BAF complex subunit ARID1B and the chromatin state of NSCs. The normal development of hippocampus requires proper spatiotemporal coordination of FGF13 isoform expression. (Image by CEBSIT)
AUTHOR CONTACT:
ZHOU Jiawei
Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, Shanghai, China.
Phone: 86.21.5492.1625; E-mail: jwzhou@ion.ac.cn 附件下载:
附件下载: