Time:2021-05-04
A recent study published in Nature Methods demonstrated that novel compact CRISPR-Cas13 systems identified from uncultivated microbes exhibited robust RNA interference and base editing activity for both mammalian and viral RNA in cultured cells. This work was performed by researchers in Dr. Yang Hui’s Lab at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, State Key Laboratory of Neuroscience and Dr. Lai Jingsheng’s team at College of Agronomy and Biotechnology in China Agricultural University.
This work has successfully identified two new CRISPR/Cas13 subtypes, Cas13X and Cas13Y, through computational analysis of large-scale microbial metagenomic data. Based on functional experiments and structure guided engineering, they developed a set of high-efficiency and high-specificity RNA editing tools from Cas13X.1 for efficient RNA knockdown and precise base editing, which is promising to be used as medicine for treating different diseases.
CRISPR/Cas13 is a type of RNA-guided ribonuclease system, which is widely used in the fields of RNA knockdown, RNA base editing, RNA site-directed modification, live cell imaging, and nucleic acid detection. Compared with the traditional RNA interference technology, the Cas13 system showed higher knockdown efficiency and specificity. Moreover, in contrast with Cas9-mediated DNA editing technology, RNA editing by Cas13 will not cause permanent changes to the genome, and can even be achieved in drugs inducible manner to make it reversible. Thus, it has unique advantages for disease treatment in the future.
In 2015, evolutionary biologist Eugene Koonin of the National Center for Biotechnology Information and Zhang Feng’s team from the Broad Institute of the United States used computational biology methods to analyze microbial metagenomic datasets and first discovered Cas13a(c2c2), Cas13b(c2c6) and Cas13c(c2c7)systems. Subsequently, Zhang Feng lab demonstrated that these Cas13 were RNA guided ribonuclease and successfully engineered to efficiently knock down target RNA in mammalian cells. Later on, Zhang Feng's team further built up RNA base editing tools from inactivated Cas13 and RNA deaminase, ADAR2. In 2018, Ph.D. graduates Patrick D Hsu and David Scott from Feng Zhang’s lab joined the Salk Institute and Arbor Biotechnologies Inc. respectively to continue working on the identification of new Cas13 systems.
Soon after Eugene Koonin and Zhang Feng’s discovery of Cas13a/b/c, they reported another Cas13d system independently on Cell and Molecular Cell respectively. Compared with the previous Cas13a/b/c, Cas13d protein was reduced by 100 to 200 amino acids. Among them, the RfxCas13d discovered by Patrick D Hsu's laboratory exhibits the best knockdown efficiency, and its small size makes it widely sought after by researchers in the field. Although RfxCas13d has only 967 amino acids, the entire system is close to the capacity limit of a single adeno-associated virus (AAV) vector, especially for Cas13-based RNA base editing tools which were difficult to deliver with only a single AAV vector. The development of novel smaller and efficient Cas13 proteins is of great significance for clinical translation of these RNA editing tools.
The Cas13 systems reported recently were all identified from cultivable microbes. However, 90% of the microorganisms in nature are not cultivable. Therefore, the Yang Hui’s team focused on mining metagenomic datasets from natural uncultivated microbes.
Their study identified two novel Cas13 subtypes through sophisticated computational algorithms and experimental design, and named them as Cas13X and Cas13Y. Among them, the Cas13X.1 protein is nearly 200 amino acids smaller than the commonly used RfxCas13d protein, which is the smallest Cas13 protein reported so far. By testing ribonuclease activity on several endogenous transcripts in mammalian cells, Cas13X.1 showed comparable high activity and specificity with RfxCas13d, and lower collateral cleavage activity than Cas13a and Cas13d. Furthermore, the research team repurpose Cas13X.1 as anti-RNA virus modality to successfully knockdown several RNA targets virus. Last but not least, Yang Hui’s team generated a series of RNA base editing tools by Cas13X.1-ADAR2dd fusion and protein truncation, screening out a mini RNA base editor.
Their results showed mini Cas13X.1 editor supported both efficient A to I and C to U conversion in target RNA. Moreover, the whole transcriptome analysis found that the mini base editor exhibited extremely low off-target activity.
This work successfully identified two novel Cas13 systems from uncultivated microbes, which greatly enriched the diversity of the Cas13 family. Furthermore, it proved by different computational, biochemical and cellular experiments that Cas13X.1 holds great potential in different application scenarios as an efficient RNA editing enzyme. It is expected to be developed as an efficient and safe RNA therapeutics in treating different diseases (especially rare diseases) in the future.
This work entitled “Programmable RNA editing with compact CRISPR-Cas13 systems from uncultivated microbes” was published online in Nature Methods on May 3, 2021. XU Chunlong, ZHOU Yingsi, XIAO Qingquan, HE Bingbing and Geng Quannan are the co-first authors with equal contribution. This work was supported by MOST, NSFC , CAS and STCSM.
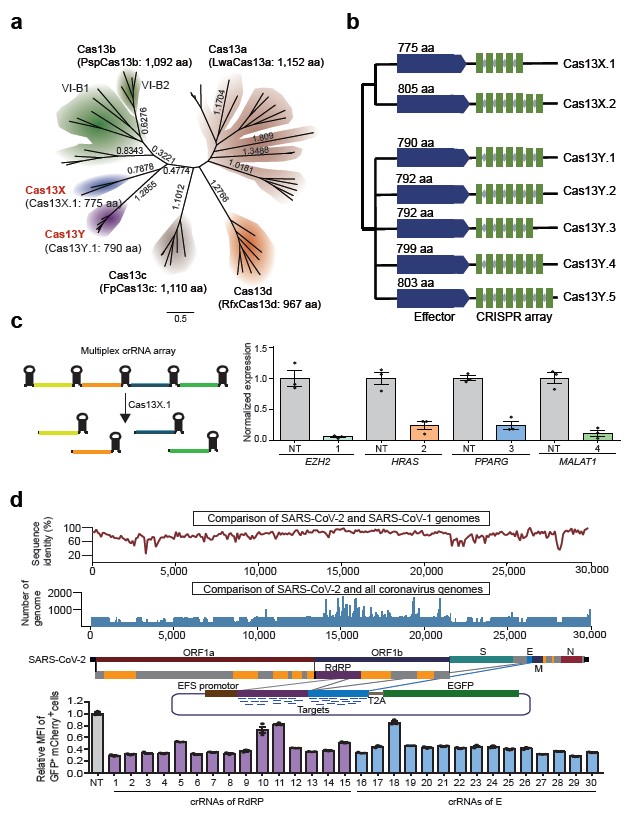
Fig. 1 Identification of novel compact Cas13 system from uncultivated microbes for efficient mammalian RNA knockdown. a, Maximum-likelihood tree of Cas13X, Cas13Y and previously reported Cas13a, Cas13b, Cas13c and Cas13d. Commonly used family members and protein sizes are shown in parentheses. The evolutionary distance scale of 0.5 is shown. b, Maximum-likelihood phylogenetic tree of Cas13X and Cas13Y proteins identified in this study, with the full Cas13X and Cas13Y CRISPR loci drawn along with conserved HEPN RNase domains. Blue and green rectangles indicate Cas13 proteins and CRISPR DRs, respectively. Gray diamonds denote spacer sequences. c, Arrays of four guides; each mediates target knockdown by Cas13X.1 in HEK293T cells via transient transfection. d, Top, comparison of sequence identity between SARS-CoV-2 and SARS-CoV-1 genomes, and alignment comparison of SARS-CoV-2 and all coronavirus genomes. Middle, schematic diagram of the reporter consists of EFS promoter, GFP and the synthesized RdRP and E fragment sequences. Bottom, GFP expression after cotransfection of the reporter and Cas13X.1/crRNA, as measured by flow cytometry. Mean GFP fluorescence intensity changes of the reporter caused by 30 different targeting crRNAs, relative to nontargeting (NT) crRNA. (Image by CEBSIT)

Fig. 2 Engineering mini RNA base editors from Cas13X.1 for efficient A-to-I and C-to-U conversion. a, Schematic procedure of testing eABE with the mCherry* reporter system. b, Predicted protein structure of Cas13X.1 and ADAR2dd. Yellow denote N terminal; green indicate C terminal; red represent HEPN motif; orange depicts ADAR2dd. c, The activity of a variety of truncated xABE variants was analyzed by reporter assay. The black bar on Cas13X.1 indicates the HEPN domain. d, A-to-I editing efficiency of xABE, mxABE and REPAIR_v2 on endogenous transcripts in HEK293T analyzed with deep sequencing. e, C-to-U editing efficiency of xCBE, mxCBE and RESCUE_S on endogenous transcripts in HEK293T analyzed with deep sequencing. f, Manhattan plots of transcriptome-wide off-target RNA editing analysis for GFP/mCherry (control), xABE, mxABE, xCBE and mxCBE transfection experiments in HEK293T cells (A-to-I editor targeting endogenous SMAD4 RNA; C-to-U editor targeting endogenous PPIB RNA). The x and y axes are proportionally enlarged with each Manhattan plot to make the axis legend clear. Non-DR, guide RNA without DRs; NT, nontargeting crRNA. All values are presented as mean ± s.e.m (n = 3). (Image by CEBSIT)
AUTHOR CONTACT:
YANG Hui
Center for Excellence in Brain Science and Intelligence Technology (Institute of Neuroscience), Chinese Academy of Sciences, Shanghai, China.
E-mail: huiyang@ion.ac.cn
 附件下载:
附件下载: