Time:2020-04-06
A recent study published in Cell revealed that glia-to-neuron conversion alleviates symptoms of two top-ranked neurological diseases in mice. This work was performed by researchers in Dr. YANG Hui’s Lab at the Center for Excellence in Brain Science and Intelligence Technology, Institute of Neuroscience, State Key Laboratory of Neuroscience, Chinese Academy of Sciences. This study showed that knockdown of a single gene Ptbp1 using a recently developed RNA-targeting CRISPR system CasRx, resulted in the conversion of Müller glia (MG) into retinal ganglion cells (RGCs) in mature retinas, leading to the restoration of visual responses in a mouse model with permanent vision impairment. Using a similar approach, it demonstrated that knockdown of Ptbp1 in the striatum locally induced neurons expressing dopaminergic markers with a very high efficiency and alleviated motor dysfunctions in a mouse model of Parkinson’s disease (PD). Together, this study provides a new approach for treating a variety of disorders due to neuronal loss.
The human nervous system contains hundreds to thousands of different types of neurons. In the mature nervous system, it is commonly accepted that neurons could not proliferate and be regenerated after injury. Thus, the death of neurons may result in the occurrence of different neurodegenerative diseases, such as Alzheimer’s disease and PD. Currently, the pathogenesis of these diseases remains unclear and no cure exists. It was estimated that more than 100 million people worldwide are living with neurodegenerative diseases and the number will increase with the increasing aging population. Among common neurodegenerative disorders, RGC death-induced permanent blindness and PD are two special ones, which are caused by the death of specific types of neurons. The reason why we could see the outside world is because there is an intact visual pathway between the eye and brain. Retinal ganglion cells (RGCs), the sole output neurons of the retina, are very sensitive to the harmful stimuli. Based on previous reports, RGC degeneration occurs in many ocular diseases such as glaucoma, which is the leading cause of permanent blindness (more than 10 million worldwide). PD, the second most common neurodegenerative disease, is characterized by the progressive death of dopamine neurons in the substantia nigra, leading to a reduction of dopamine concentration in the striatum. It was estimated that close to 10 million people are affected by this disease globally and nearly half of the population are in China. Regeneration of these two specific types of neruons in the adult nervous system is a world-class problem and the aim of many scientists.
To specifically downregulate Ptbp1 expression in the retinal MG with a high efficiency, the authors first screened six guide RNAs (gRNAs) for their efficiency in CasRx editing of
Although this is an important breakthrough, it should be noted
This work entitled “Glia-to-Neuron Conversion by CRISPR-CasRx Alleviates Symptoms of Neurological Disease in Mice” was published online in Cell on April 8, 2020. Dr. ZHOU Haibo, Dr. SU Jinlin, HU Xinde, ZHOU Changyang, LI He and CHEN Zhaorong contributed equally as the first authors. Dr. YANG Hui and Dr. Zhou Haibo are corresponding authors. This work was also strongly supported by Dr. POO Mu-ming, Dr. XU Huatai, Dr. ZHANG Yifeng, Dr. YAO Haishan and Dr. ZHOU Jiawei, and CEBSIT core facilities: genome editing facility, optical imaging core facility, mice facility and FACS facility. This work was funded by the Ministry of Science and Technology of the People′s Republic of China, Chinese Academy of Sciences, Science and Technology Commission of Shanghai Municipality.
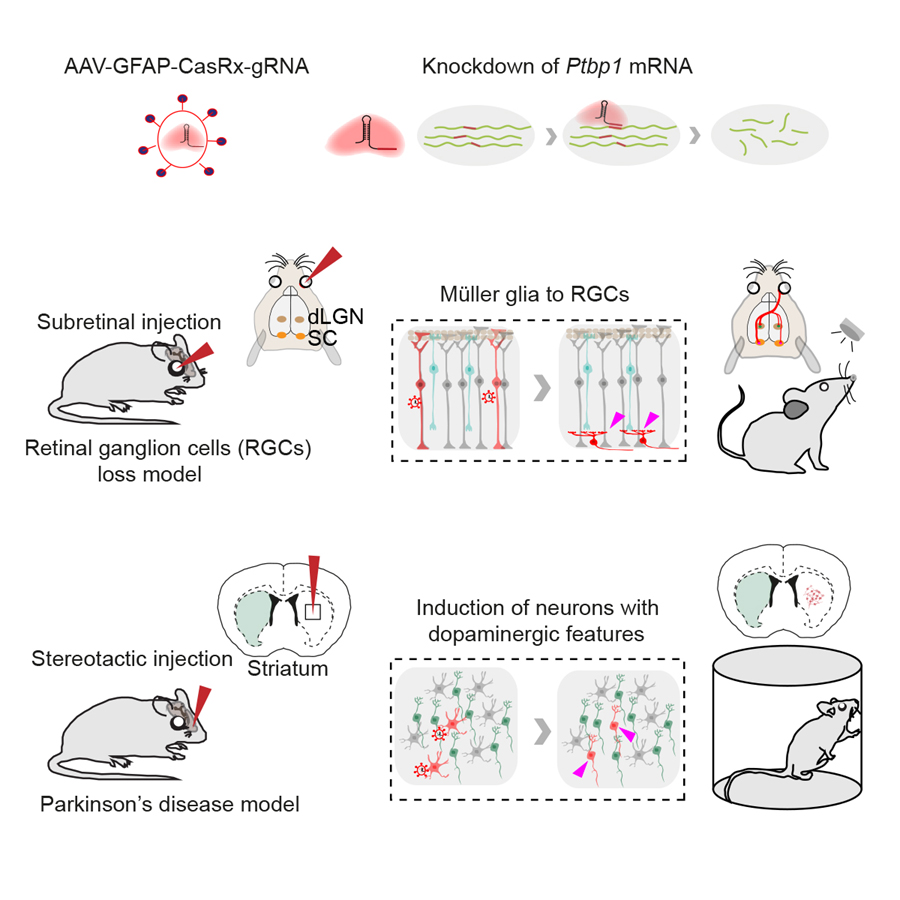
Legend: (Upper) CasRx-mediated knockdown of Ptbp1 mRNA. (Middle) Subretinal injection of AAV-GFAP-CasRx-Ptbp1 specifically converted MG into RGCs in the mature retinas and restored visual responses in NMDA-injured mice. (Lower) Stereotactic injection of AAV-GFAP-CasRx-Ptbp1 converted astrocytes into neurons with dopaminergic features in the striatum and alleviated motor dysfunctions in PD mice.
Contact: YANG Hui
Center for Excellence in Brain Science and Intelligence Technology, Institute of Neuroscience, Chinese Academy of Sciences
Email:huiyang@ion.ac.cn
 附件下载:
附件下载: