Time:2019-12-13
The ability to control the expression of any interested genes at will has been a dream of many biologists. CRISPR/Cas9, known as a "molecular scissor", cleaves the DNA double-strand with high specificity. Recently, a catalytically dead Cas9 (dCas9) was developed as a powerful alternative. Targeted activation of endogenous genes can be achieved by fusing dCas9 with transcriptional activators. dCas9-based transcriptional activation has many advantages over traditional "overexpression" methods. It is not constrained by the size limit of inserted open reading frame (ORF) sequence in the viral expression vectors, thus multiplex activation can be easily achieved. Moreover, it can accurately model the expressions of the complex transcript isoform variances. dCas9-mediated activation has been verified and widely used in different cell lines in vitro. However, in vivo applications of dCas9-activator remain unverified.
In this study, researchers designed an improved activator SPH, which showed more potent activation efficiency in both human and mouse cell lines compared with different representative ‘second generation’ activators. Then the researchers generated a Cre-dependent SPH transgenic mouse, and showed that genes and LncRNAs could be activated in primary cells after infection of lentivirus expressing sgRNAs and Cre recombinase. To determine the feasibility of using SPH mice to induce transcriptional activation in vivo, sgRNAs plasmids were delivered to the liver by hydrodynamic injection, demonstrated that genes could be efficiently upregulated in vivo. Especially, via targeted activation of theDkk1 gene, which encodes a Wnt antagonist, metabolic zonation of the liver was remodeled. The researchers further showed that mature astrocytes in the midbrain can be converted into functional neurons by injecting AAV targeting three previously described neurogenic transcription factors:Ascl1, Neurog2 and Neurod1, confirmed that SPH transgenic mice can be used to modulate neuronal functions in vivo. Finally, the researchers injected sgRNA arrays targeting ten genes or ten genetic elements including eight genes and two lncRNAs into the brain. As expected, most of the targets were potently upregulated. Collectively, these results highlight the advantages and potential of using SPH mice to modulate complex genetic networks in the intact brain.
This work entitled “In vivo simultaneous transcriptional activation of multiple genes in the brain using CRISPR–dCas9-activator transgenic mice” was published online in Nature Neuroscience on January 15, 2017. ZHOU Haibo, LIU Junlai, ZHOU Changyang, GAO Ni, RAO Zhiping, LI He are the first authors with equal contribution. This work was supported by 2017YFC1001302, CAS Strategic Priority Research Program, the MoST863 Program, NSFC grants, Break through project of Chinese Academy of Sciences, Shanghai Sailing Plan for the Young Scientific Talents, and The Ministry of Science and Technology of China.
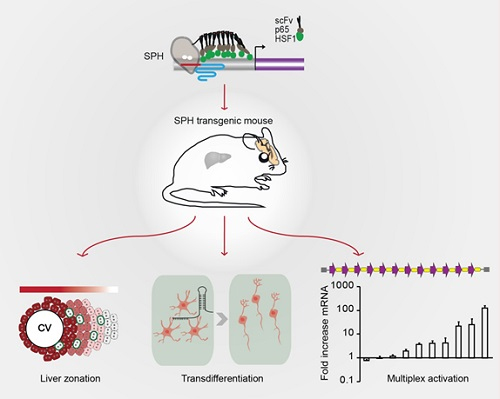
Graphic Abstract: This study developed an improved activator SPH and generated a Cre-dependent SPH transgenic mouse. The researchers demonstrated that SPH transgenic mice could be used to remodel metabolic zonation of the liver, directly convert astrocytes into functional neurons and simultaneously activate multiple genetic elements in the brain.
 附件下载:
附件下载: