Time:2021-12-06
Two recent studies published in The Journal of Neuroscience and National Science Review characterized the coding mechanism of multimodal somatosensations in the primary somatosensory cortex (S1), and revealed the neural mechanism of itch perceptual coding in S1. These studies were performed by researchers in Dr. SUN Yangang’s lab and Dr. XU Ninglong’s lab at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, State Key Laboratory of Neuroscience. These studies have successfully delineated the cortical coding mechanism of itch in free-moving animals, and has paved the way for a deeper understanding of the processing and integration of multimodal somatosensory information in the cortex.
Somatosensation is crucial for many physiological processes. It includes many different submodalities, such as pain, temperature, touch etc. Cortical processing of somatosensory information is one of the most fundamental aspects in cognitive neuroscience, but neural mechanisms underlying the coding of multiple somatosensations remain largely unknown. Itch represents another submodality of somatosensation, serving as an important protective mechanism. However, cortical representation of itch perception remains unclear.
Previous studies found that primary sensory afferents exhibited complicated response patterns to different somatosensory stimuli, and multiple somatosensory stimuli, including thermal, tactile, pruritic, and nociceptive stimuli, could activate the same neuronal population in the spinal cord. These results further raised the question of how the nervous system decodes multiple submodalities of somatosensation. Besides, most studies examining the coding mechanisms of itch were conducted in anesthetized animals, which could not report the itch perception by scratching behavior. Thus, it remains to be determined whether and how S1 encodes itch perception.
To address the question of how S1 encodes different somatosensations, researchers adopted the in vivo two-photon calcium imaging in S1, while delivering pruritic, touch, or thermal stimuli in mice. Researchers found that S1 neurons reliably responded to touch and thermal stimuli. With a newly-established optogenetics-based paradigm that allows precisely-controlled pruritic stimulation (opto-itch), researchers found that S1 encodes the spatiotemporal and intensity aspects of itch sensation. Moreover, they found that touch, thermal, and pruritic stimuli activated largely overlapping neuronal populations in S1. Population decoding analysis revealed that the local neuronal population in S1 encoded sufficient information to distinguish different somatosensory submodalities.
To examine the perceptual coding capability of S1 in a natural itch perceptual task, researchers took advantage of a novel miniature two-photon microscope to perform calcium imaging in free-moving mice, while applying chemical pruritic stimuli. They found that the itch-induced scratching behavior could be well predicted by the activity of a fraction of S1 neurons, suggesting that a subpopulation of S1 neurons encoded itch perception. With the newly-established opto-itch paradigm, researchers found that a small fraction of S1 neurons exhibited ignition-like pattern at the detection threshold of itch perception.
These studies demonstrated the representation scheme of different somatosensations in S1, offering a new perspective for a deeper understanding of the processing and integration of multimodal somatosensory information in the cortex. Besides, it also revealed the coding mechanism of itch perception in S1, paving the way for studying cortical representation of itch perception at the single-neuron level in free-moving animals. Furthermore, the newly-established opto-itch paradigm also provides a methodological basis for further investigations of itch.
These two research articles entitled “Multiplexed representation of itch and mechanical and thermal sensation in the primary somatosensory cortex” and “Itch perception is reflected by neuronal ignition in the primary somatosensory cortex” were published online in The Journal of Neuroscience and National Science Review on October 29 and December 3, 2021. This research was mainly conducted by graduate student CHEN Xiaojun under the supervision of Dr. SUN Yangang and Dr. XU Ninglong, and graduate student LIU Yanhe also made important contributions. These works were supported by the National Natural Science Foundation of China, Chinese Academy of Sciences, and the Shanghai Municipal Government.
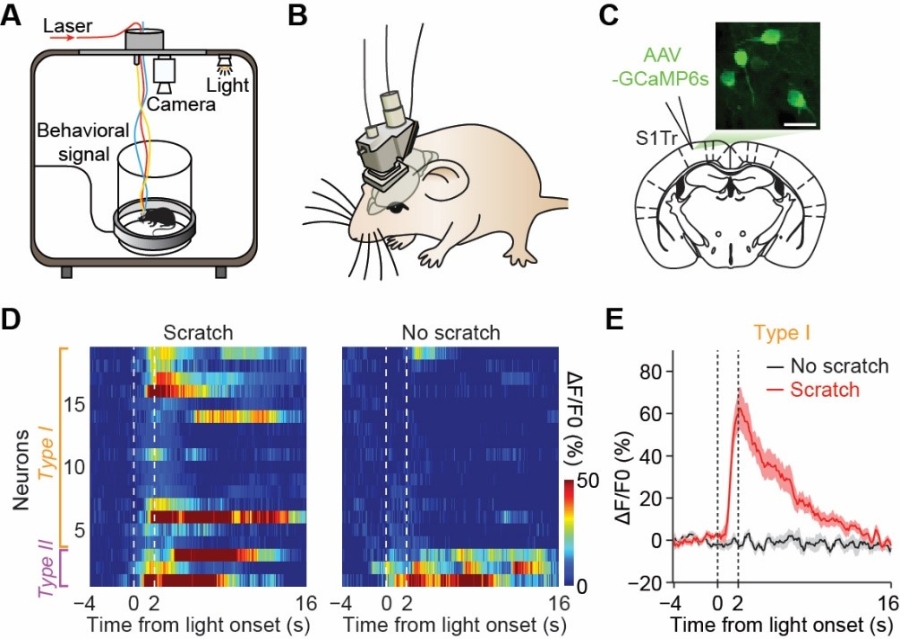
Figure legend: (A) Schematic of simultaneous calcium imaging and scratching behavior recording in free-moving mice. (B) Schematic of miniature two-photon microscope mounted on a mouse head. (C) Image of GCaMP6s-labeled neurons in S1. (D) Two types of S1 neurons exhibiting different response patterns to threshold opto-itch stimuli. (E) Example neuron showing ignition-like activation pattern.
AUTHOR CONTACT:
SUN Yangang
Center for Excellence in Brain Science and Intelligence Technology (Institute of Neuroscience), Chinese Academy of Sciences, Shanghai, China.
E-mail: yangang.sun@ion.ac.cn
 附件下载:
附件下载: