

Time:2020-08-06
Neural recording and manipulation
Multi-channel single-unit recording
Rabies based trans-synaptic tracing
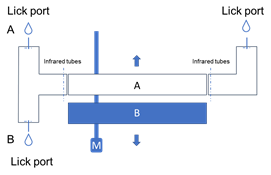
· Fully automatic control of behavioral training
· Interleaved trials for context switches
· Context-dependent tasks in behaving animals

· Rodent running on a spherical treadmill
· 270° TFT monitor (or 150° curved back-projecting screen) for presenting virtual reality
· Custom behavioral training with licking reward and air-puff punishment
· Compatible with 2-photo microscopy imaging
· Compatible with miniscope Ca2+ imaging


· Custom software to acquire behavioral video and trigger optogenetic devices
· Compatible with photometry and miniscope recording


· 64 channel acquisition systems from BlackRock and Open-Ephy
· Tetrode recording in free-moving animals
· Spike sorting using OFSS


· > 1mm diameter field of view and ~ 1mm working distance
· +/- 200um electronic focal adjustment
· 2.6 grams and 22 mm tall
· Absolute head orientation sensor

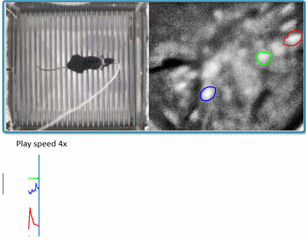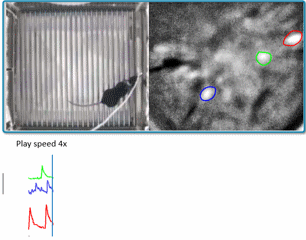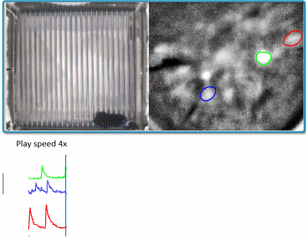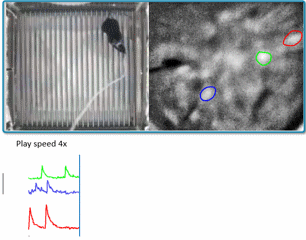
(Courtesy of Miniscope-v4 wiki. We are using v2 in the lab for now)
· Multi-Fiber Photometry System (4 channels)
· Integrated with behavioral video acquisition and analysis
· Synchronized with external equipment (such as optogenetics) via TTL
· Axon 700A/700B, Digidata 1440, pClamp10.
· Sutter Motorized Micromanipulator, Nikon microscope, Thorlab CCD.



· npi amplifier ELC-03XS, CED Micro 1401


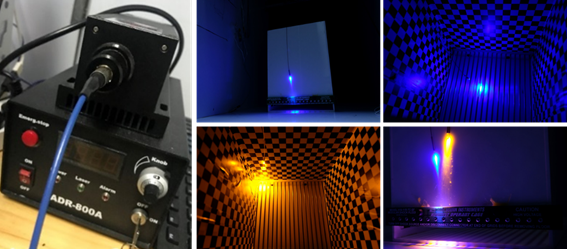
· 462 nm diode blue laser, 589 nm DPSS yellow laser
· Adjustable power, TTL trigger mode
· Blue, Green, Red and Far-red channels
· Equipped with motorized platform and NeuroLucida 8

· Digital display of stereotaxic coordinates
· Precise control of isoflurane
· Fast anesthesia and fast recovery



· Standard equipment for cloning


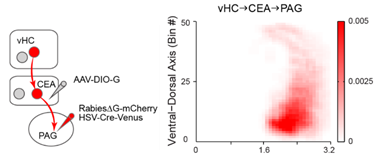
· Cell-type specific retrograde trans-synaptic tracing
· Example below showing projection-cell-specific tracing of vHC-CEA-PAG pathway
· Quantitative analysis of circuit tracing in the whole-brain scale
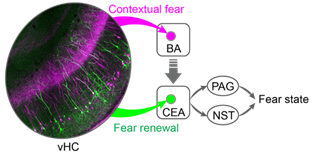
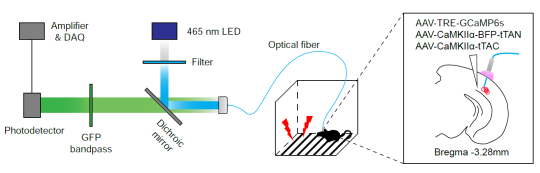
· IBIST (intein based intersectional synthesis of transactivator)
· Intersectional expression for ChR2 and GCamP6 in tTA-dependent manner
 附件下载:
附件下载: