Time:2014-11-18
Mechanical sensations, including touch sensation, sound sensation, proprioception and mechanical nociception, are essential for animal survival, but the molecular mechanisms underlying mechanical transduction are just beginning to be elucidated. It has long been known that mechanoreceptors respond to forces through mechano-gated ion channels since the responses are too rapid to involve second-messenger cascade. Only recently, however, promising ion channel candidates, including only a few members of the TRP, DEG/ENaC and Piezo families, have been identified.
To date, 31 members of the DEG/ENaC channel family have been identified in the Drosophila melanogaster genome. However, only PPK and RPK have been implicated in mechanosensation. To reveal novel DEG/ENaC channels that participate in mechanosensation, graduate students GUO Yanmeng and WANG Yuping from Dr. WANG Zuoren’s lab at the Institute of Neuroscience, Chinese Academy of Sciences, first performed phylogenetic analysis of all the Drosophila DEG/ENaC channels and then examined the expression pattern of several candidates. Combining use of the Gal4/UAS system, functional silencing and immunostaining studies, they found that PPK26 are selectively expressed in class IV dendritic arborization (da) neurons.
The class IV da neurons, which detects intense mechanical forces and harmful heat as well as intense short-wave light, represents the polymodal nociceptor in Drosophila larvae. Previous work showed that PPK, Pain, and Piezo proteins all contributed to mechanical nociception in the class IV da neurons. Here, the authorsfound that PPK26 contributes specifically to mechanical nociception but not to thermal nociception in class IV da neurons, functioning together with PPK.
Genetic interactions studies indicate that PPK26 and PPK function in the same pathway in mechanical nociception while Piezo functions in a parallel pathway. In consistent with this result, the authors found that PPK and PPK26 are interdependent on each other for their plasma membrane localization, while Piezo do not affect their plasma membrane localization. This work might provide new clues to future studies on mechanical nociception in mammals.
This research entitled “The Role of PPK26 in Drosophila Larval Mechanical Nociception” was published online in Cell Reports on November 06, 2014. The work was supported by the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (grant no. XDB02010005) and China 973 Project (grant no. 2011CBA0040) to Z.W. The authors gratefully acknowledge the support of SA-SIBS scholarship program.
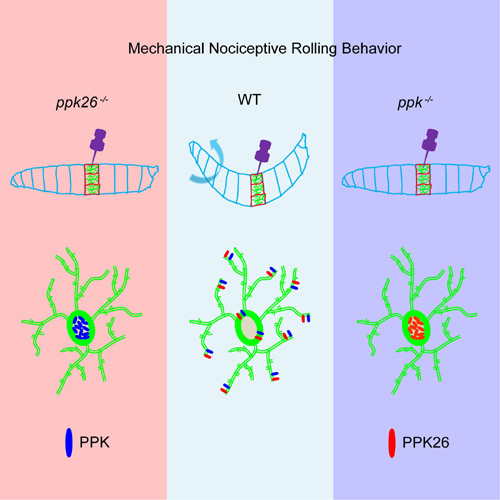
Left: ppk26 mutant larvae exhibit remarkably reduced rolling behavior in response to mechanical noxious stimuli and PPK could not translocate to the plasma membrane.
Middle: Wild type larvae respond to mechanical noxious stimuli with rolling behavior.
Right: ppk mutant larvae exhibit remarkably reduced rolling behavior in response to mechanical noxious stimuli and PPK26 could not translocate to the plasma membrane.
 附件下载:
附件下载: