Time:2022-03-22
A recent study published in Cell Reports revealed the single-cell transcriptome characteristics of mouse otic neuronal lineage at three different embryonic stages E9.5, E11.5 and E13.5, and discovered a variety of new genes specific for otic precursor and for inner ear ganglion subtypes. The results of this study provide a new theoretical basis for the treatment of balance and auditory dysfunction caused by abnormal inner ear ganglia. This work was performed by researchers in LIU Zhiyong’s lab at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology (CEBSIT) of the Chinese Academy of Sciences (CAS), and the research group of WEI Wu at the Shanghai Institute of Nutrition and Health (SINH), CAS.
The vestibular ganglion neurons (VGNs) and spiral ganglion neurons (SGNs) of the inner ear play key roles in maintaining body balance and perceiving sound information, respectively, and their damage or degeneration leads to balance disorders and deafness. SGNs are also important for the clinical efficacy of cochlear implants. Although the mature inner ear VGNs and SGNs have distinct functions, they both originate from the otic neuroblasts, also known as the cochlear-vestibular ganglion (CVG) precursor cells. There are currently three important questions to be addressed in the field: 1) How are inner ear CVG cells specified from the otic vesicle? 2) How do undifferentiated CVG cells gradually differentiate into VGNs and SGNs? 3) How many subtypes are there in the VGNs and SGNs at early embryonic ages? Apparently, addressing these questions will not only help to uncover the molecular mechanisms underlying the development of the inner ear ganglia, but also provide potential key genes or gene networks for regeneration and repairment of adult inner ear ganglia.
Using 10x Genomics single-cell RNA-sequencing technology, this study revealed the transcriptome of mouse inner ear tissue at three early embryonic stages (Fig. 1A), and found that as neural differentiation progressed, CVG cells migrate from the otic epithelium and gradually lose their epithelial characteristics (Fig. 1B). At E9.5, a subset of Neurog1+ otic vesicle cells begin to express Insm1 and Shox2, eventually become CVG cells. VGNs and SGNs start to exhibit distinct gene profiles at E11.5. In addition, the findings reveal that during otic neurogenesis, the production of undifferentiated CVG cells and the differentiation of VGNs or SGNs occur concurrently (Fig. 1D, Fig. 2). According to the newly discovered genes specific for different stages of CVG precursors, VGNs and SGNs, they constructed two new transgenic mouse models of Shox2-P2A-Cre/+ and Casz1*3xHA-P2A-Tdtomato/+, which can be used for follow-up more accurate inner ear ganglion labeling and gene function analysis.
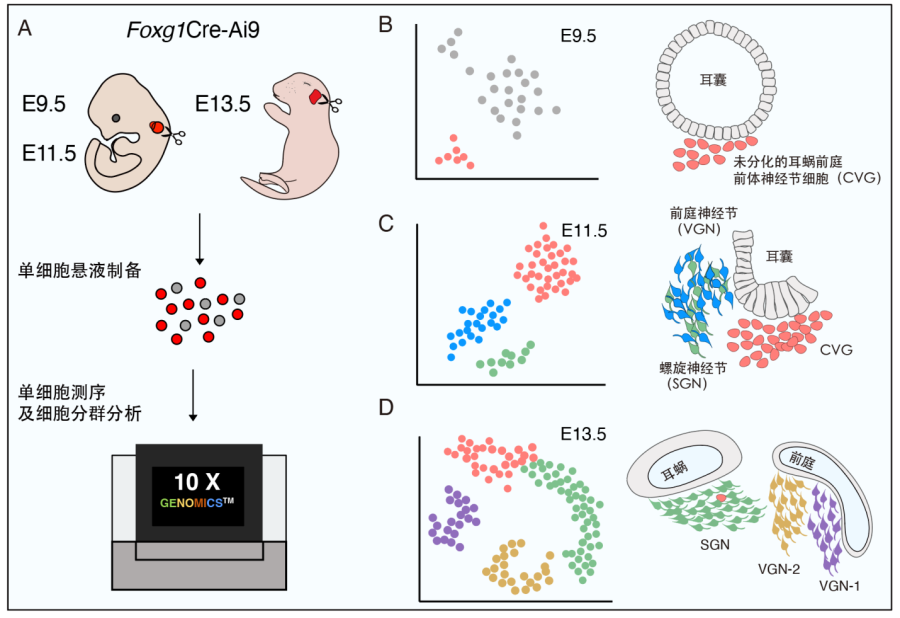
Figure 1. Transcriptome analysis of the otic in the early mouse embryo. (A)Schematic diagram of 10x Genomics single-cell sequencing experiments in E9.5, E11.5 and E13.5 inner ear tissues. (B)E9.5 otic cells can be divided into epithelial cell populations (grey) and cochleo-vestibular ganglion neurons (CVG, pink). (C)E11.5 otic neural lineage cells are divided into three populations, differentiated vestibular ganglion neuron (VGN, blue) and spiral ganglion neuron (SGN, green) and undifferentiated CVG cells (pink). (D) Inner ear neurons at E13.5 are divided into undifferentiated CVG cells (pink), SGN (green), type I VGN (purple), and type II VGN (yellow).
Previous studies revealed the existence of four distinct subtypes in adult SGNs. The trajectory analysis in this study suggested that the SGN subtypes have not been established at E13.5. Conversely, this study identified two subtypes of VGN at E13.5 (Fig. 2): Tlx3 +/Sall3+/Gata3- type I VGN and Tlx3+/Sall3-/Gata3+ type II VGN, therefore, the VGN subtypes appear earlier than the SGN counterparts. The specific functions of these VGN subtypes will be an important research direction in future.
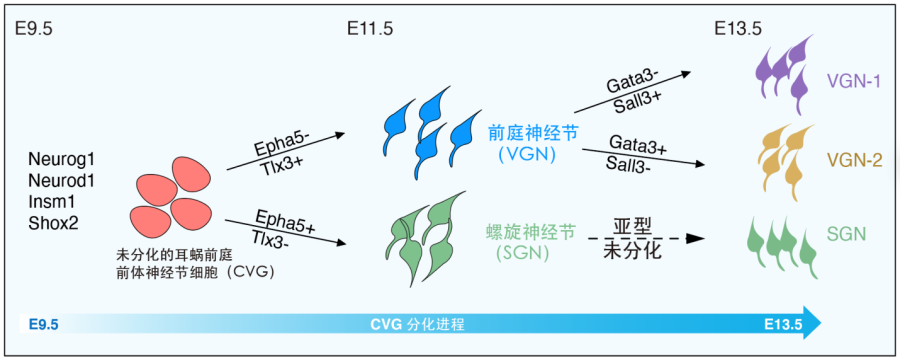
Figure 2. Diagram of development and differentiation of cochleo-vestibular ganglion (CVG) cells.
This research entitled ‘Single-cell transcriptomic landscapes of the otic neuronal lineage at multiple early embryonic ages’ was published online in the journal of Cell Reports on March 22nd, 2022. Dr. LIU Zhiyong, Dr. LI Chao from CEBSIT, and Dr. WU Wei from SINH are the co-correspondences of this work. Graduate Students SUN Yuwei from CEBSIT and WANG Luyue from SINH are the co-first authors of this paper. Research assistants WU Bailin and ZHU Tong, Graduate Students WANG Guangqin and LUO Zhengnan from CEBSIT also made important contributions to this project. The research has been supported by the Molecular and Cellular Biology Core Facility, Optical Imaging Core Facility, and Animal Facility (Mice Facility) of CEBSIT, CAS. The research was supported by the Ministry of Science and Technology, the Chinese Academy of Sciences, the Natural Science Foundation of China, and Shanghai municipal.
 附件下载:
附件下载: