Time:2021-03-09
Recently, Dr. LIU Zhiyong’s group from the Institute of Neuroscience (ION), State Key Laboratory of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology (CEBSIT) of the Chinese Academy of Sciences (CAS), established a rapid genetic screening method. This study highlights that mosaic CRISPR-stop is a reliable method and will pave the way for genetic screening of developmentally essential genes in the mouse inner ear and other organs.
There is a high degree of similarities regarding the developmental process and functions of auditory system between mice and human beings, especially sound receptor cells, hair cells (HCs). Genetic screening of key genes regulating HC differentiation is of particular importance in gene therapy toward HC restoration in deaf patients in clinic. While transcriptomic profiles of HCs at multiple postnatal ages are currently available, it remains poorly understood in terms of which genes are essential in regulating HC differentiation and maturation. One of the main reasons is that generating mutant Founder 0 (F0) mice, germ-line transmission and subsequent inter-breeding are time-consuming and laborious.
Their previous work has established a protocol, which is able to inactivate three non-lethal genes simultaneously in F0 mice which are ready for immediate phenotype analysis. However, there are 25% of mammalian genes whose homozygous mutants will lead to embryonic or neonatal lethality, thus the previous strategy is not suitable for those lethal or essential genes. The typical solution is using conditional knock out strategy, but this will take a very long time.
Is there an alternative approach to rapidly screen those essential genes? This study provided a novel genetic strategy named ‘mosaic CRISPR-stop’ to generate F0 mice with homozygous but mosaic mutations. By injecting base editor components and gene specific sgRNAs into one of the two blastomeres, offspring cells with or without gene editing are distributed randomly throughout various organs.
First, to assess efficiency of mosaic CRISPR-stop, Atoh1 is chosen for the proof-of-concept study. No HCs are produced in Atoh1 -/- mice that die immediately after birth. Atoh1 mosaic mutant mice not only survive longer, but also have much less HCs. Second, the phenotype of shortened cochlea in Sox10 mutants is also reproduced by mosaic CRISPR-stop. Third, Rbm24 is another essential gene expressed in cochlear HCs and Rbm24 -/- mice die due to heart dysfunction at embryonic ages. With mosaic CRISPR-stop, we show that HCs are initially produced, but gradually die in the absence of Rbm24. Such a HC death phenotype is able to be validated by the typical Rbm24 conditional knockout model. Thus, Rbm24 is essential for the survival of HCs.
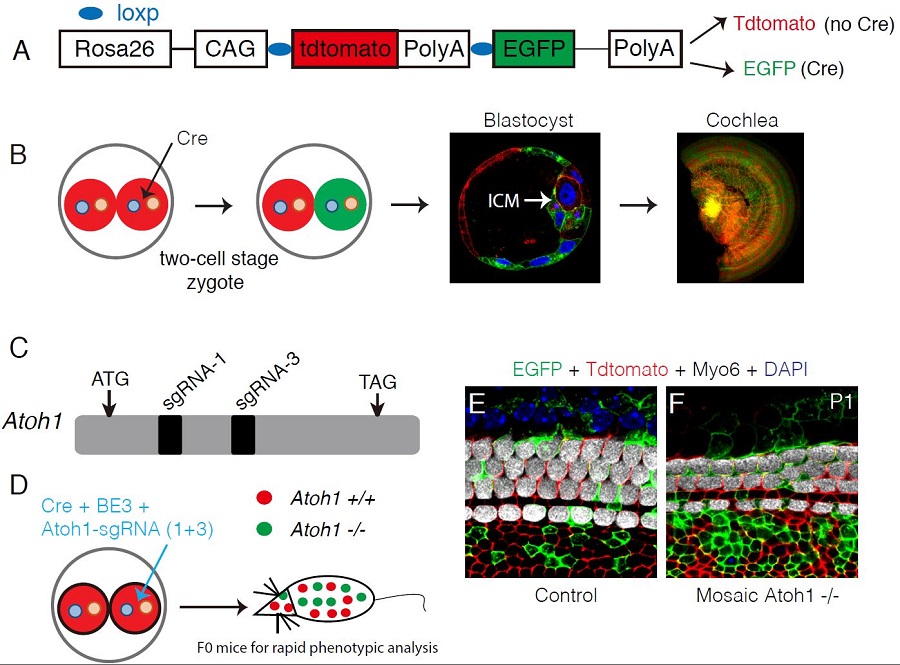
Fig. 1 Proof-of-principe study of Atoh1 using mosaic CRISPR-stop. (A) Illustration of the dual color reporter mouse. Without Cre, all cells express Tdtomato, otherwise Tdtomato is turned off and instead EGFP is on. (B) One of the two blastomeres is injected with Cre and becomes EGFP+. Progeny cells derived from either of them can develop into inner cell mass (ICM) and cochlear cells. It establishes the foundation of using mosaic CRISPR-stop for rapid genetic screening. (C) Two efficient sgRNAs are located in the single exon of Atoh1. (D) One of the two blastomeres is injected with Cre, base editor components (BE3), and two sgRNAs. (E-F) Compared with control (E), hair cell (Myo6+) number is decreased in mosaic Atoh1 -/- mice (F). (Image bu CEBSIT)
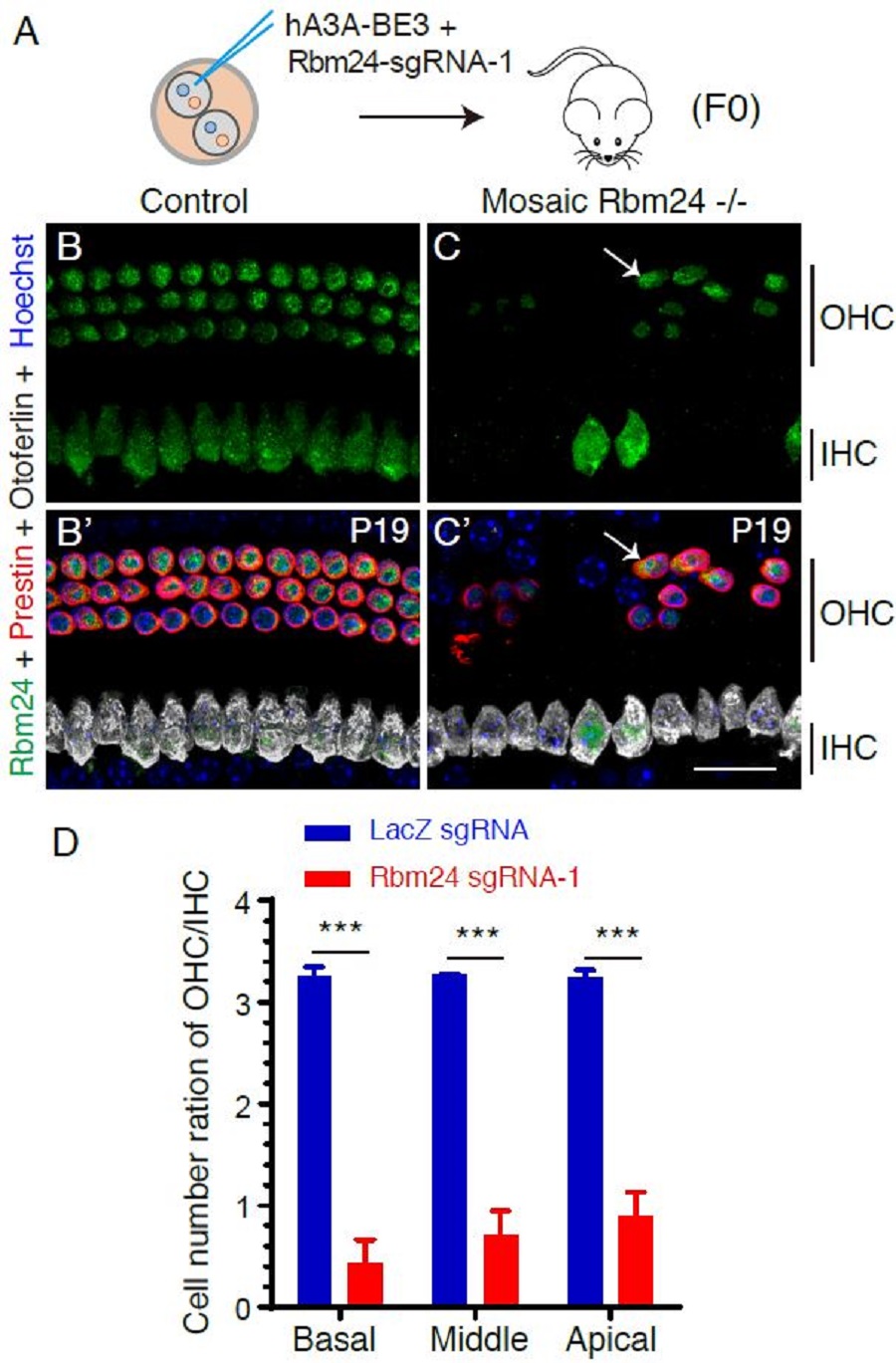
Fig. 2 Importance of Rbm24 in cochlear outer hair cells (OHCs) is rapidly determined by mosaic CRISPR-stop. (A) One of the two blastomeres is injected with updated version of base editors (hA3A-BE3) and sgRNA against Rbm24. (B-C’) Control cochlea contains one row of inner hair cells (IHCs) and three rows of OHCs (B and B’). In contrast, significant loss of OHCs occurs in mosaic Rbm24 -/- (experimental group) mice (C and C’). Arrows represent remaining OHCs in which Rbm24 is maintained. (D) Compared with control (injected with LacZ sgRNA, blue), OHC number is significantly decreased in experimental group (injected with Rbm24 sgRNA, red). (Image bu CEBSIT)
 附件下载:
附件下载: