Time:2020-05-11
A recent study published inNature Communications demonstrated that spider venom-derived peptide HpTx1 induces hyperalgesia in Nav1.7 knockout mice. This finding is from a joint work by Prof. LIU Zhonghua’s team from the College of Life Sciences, Hunan Normal University and Dr. LIU Jing Yu’s lab at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences and College of Life Science and Technology, Huazhong University. In this study, they combined genetic approaches, behavioral assessments, and patch-clamp technique. This work demonstrated that HpTx1 inhibited Nav1.7, and activated Nav1.9, but did not affect Nav1.8. In addition, it could restore the pain sensation of the Nav1.7-knockout mice. This work revealed that the activation of the Nav1.9 channel could partially compensate for the loss of function of Nav1.7 in dorsal root ganglion neurons, and characterized the relationship between the three channels. This work provided a new direction for further treatment of Nav1.7-related congenital insensitivity to pain.
Pain is the sensation of the body when subjected to various harmful stimuli. Pain is also a defensive mechanism that protects the body from injury, and can promote the healing of damaged tissues. However, the loss-of-function mutations in Nav1.7 lead to congenital insensitivity to pain. The treatment of the disease is a major challenge.
Nav1.7 deficiency leads to congenital insensitivity to pain. Previous studies have shown that endogenous opioids are increased in Nav1.7-knockout mice, while naloxone reduces endogenous opioids and restores the pain sensation. But Nav1.7 appears to be dispensable in rats. Therefore, they will explore new specific targets for treatment of Nav1.7-related congenital insensitivity to pain.
In this study, they screened and identified the spider toxin that could restore pain sensation of the Nav1.7-knockout mice. They found that the toxin could increase the current of Nav1.9, but inhibit the current of Nav1.7. Nav1.7-knockout mice, Nav1.8-knockout mice, Nav1.9-knockout mice and Nav1.7/Nav1.8-double knockout mice were used to demonstrate the role of Nav1.9 in the dorsal root ganglion neurons. They discovered the mechanism and the contribution of the three channels to action potential generation.
This work provided a new strategy and a new target for the treatment of Nav1.7-related congenital insensitivity to pain, and provided a new idea for the development of drugs. The Nav1.7, Nav1.8 and Nav1.9 can precisely regulate neuronal excitability. The activation of Nav1.9 can partially compensate for the functional deficiency of Nav1.7. Spider toxin-HpTx1 is the first agonist identified that targets the Nav1.9 channel. These results supported that Nav1.9 plays an important role in pain signaling pathway.
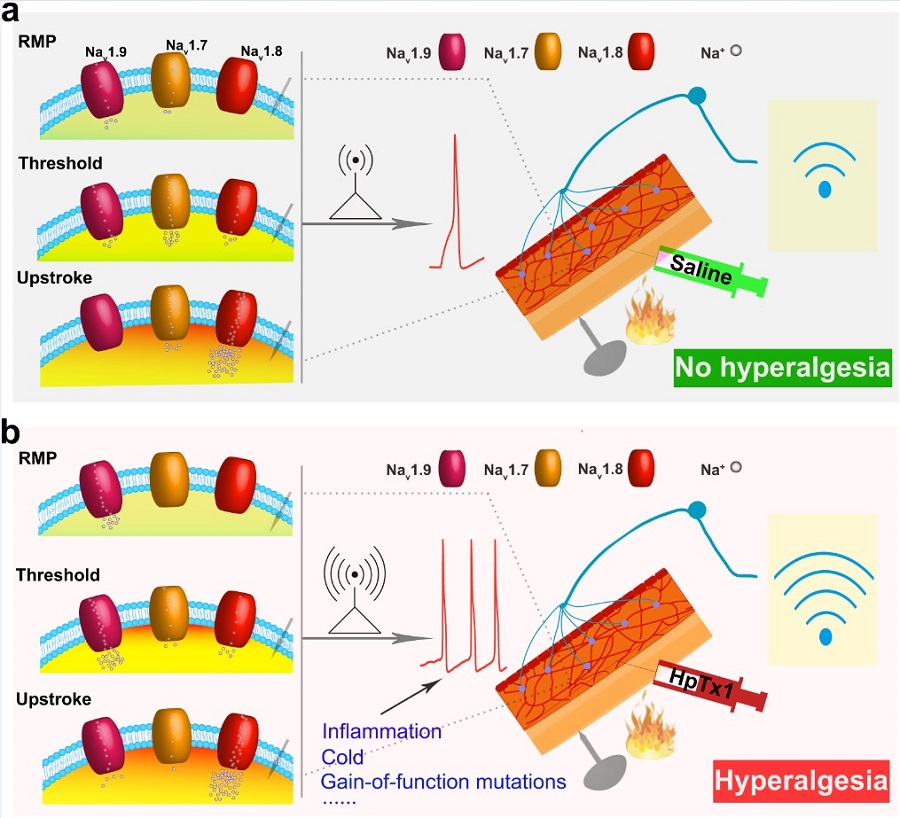
Figure legend: (a) The role of Nav1.7, Nav1.8, and Nav1.9 in the generation of action potentials under normal conditions. (b) The role of Nav1.7, Nav1.8 and Nav1.9 in the generation of action potentials after HpTx1 treatment . (Image by CEBSIT)
ZHOU Xi (College of Life Sciences, Hunan Normal University), MA Tingbin (College of Life Science and Technology, Huazhong University of Science and Technology), who were mentored by Prof. LIU Zhonghua and Dr. LIU Jing Yu, respectively, contributed equally to this work. Other members in the lab actively participated in this work, and Prof. ZHANG Xianwei (Department of Anesthesiology, Tongji Hospital of HUST) also provided great help.
In addition, Dr. LIU Jing Yu’s lab and Prof. Shi Xiaoliu’s team (Department of Medical Genetics, The Second Xiangya Hospital, Central South University) collaborated another work entitled “Alcohol-aggravated episodic Pain in humans with SCN11A mutation and ALDH2 polymorphism”. They found that the cause of pain triggered by alcohol intake was that the patients carried both Nav1.9 mutation and a SNP locus of ALDH2 (p.glu504lys). The ALDH2 polymorphism (c.1510G>A/p.Glu504Lys) was known to lead to a decrease in the enzymatic activity to 6% of the wild type. This work successfully constructed the mouse Nav1.9-KI pain model, and aldehydes were found to aggravate pain sensation in Nav1.9-KIA796G mice. It was further found that COX2 inhibitor could effectively relieve pain hypersensitivity in the Nav1.9-KI mice. Thus COX2 inhibitor might exert analgesic effect in patients with Nav1.9 mutation. This work was published online in Pain on April 7, 2020
This work was supported by the National Science Foundation of China grants and the National Key R&D Program of China.
AUTHOR CONTACT:
LIU Jing Yu
Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences
E-mail: liujy@ion.ac.cn
 附件下载:
附件下载: