Time:2020-02-25
A recent study published in Nature reported two conserved epigenetic regulators as novel anti-aging targets. This work was performed by researchers in Dr. CAI Shiqing’s Lab at the Center for Excellence in Brain Science and Intelligence Technology, Institute of Neuroscience, State Key Laboratory of Neuroscience, Chinese Academy of Sciences, and Dr. JIANG Lubing’s team at the CAS Key Laboratory of Molecular Virology and Immunology, Institut Pasteur of Shanghai, Chinese Academy of Sciences. The work has unraveled conserved negative regulators of healthy aging by using multiple modalities and systems, providing insights into how to achieve healthy aging.
Aging is associated with progressive decline in physiological functions over time and is a major risk factor for a number of chronic diseases, such as Alzheimer's disease, cancer, and diabetes. Over the past decades, our understanding on longevity regulation has progressed greatly; a number of longevity pathways conserved from yeast to mammals have been delineated. However, increasing longevity is not often accompanied by an extended healthspan, although life expectancy increases globally. Thus, how to achieve healthy aging (extension of healthspan) is one of the most important and challenging heath issues nowadays. Despite the extreme importance, the biological mechanisms underlying healthy aging, as defined by the preservation of normal behavioral capabilities, remains to be elucidated.
Previous studies from Dr. CAI Shiqing’s lab has revealed that behavior performance in aged animals could be improved by increasing neurotransmitters (Yin et. al. Journal of Neuroscience, 2014) and variation in levels of neurotransmitters may contribute to different rates of age-related decline among individuals (Yin et. al. Nature, 2017). In this study, the researchers used animal models and human datasets to identify novel anti-aging targets and unravel a regulatory mechanism of cognitive aging. C. elegans is a tiny free-living nematode, about 1 mm in length. Due to its short lifespan and clear genetic background, C. elegans has been widely used in aging research. To identify aging modulators, the researchers performed a genome-wide RNAi screen for genes that regulate behavioral deterioration in aging C. elegans. They identified 59 genes that potentially regulate the rate of age-related behavioral deterioration. By constructing a co-expression network of these screening hits, they found that a neuronal epigenetic reader BAZ-2 and a neuronal histone 3 lysine 9 (H3K9) methyltransferase SET-6 appeared as a key node in the network. Deletion of BAZ-2 and SET-6 prevented age-related deterioration in the worm’s food-induced behavior, food intake, and male virility. By analyzing published databases, the researchers found that the expression levels of their human homologues BAZ2B and EHMT1 increase with age in human brains, and positively correlate with Alzheimer's disease (AD) progression. Strikingly, ablation of Baz2b, the mouse ortholog of BAZ-2, attenuated age-dependent body weight gain and prevented cognitive decline in aging mice. Their findings suggest that BAZ2B and EHMT1 are key aging modulators and appear to be novel anti-aging targets.
They further demonstrated that these epigenetic modulators repressed the expression of nuclear genes encoding mitochondrial proteins by occupying at the promoter regions, and hence reduced mitochondrial functions, a mechanism conserved in mouse brain tissues. Deletion of baz-2/BAZ2B and set-6/EHMT1 delayed aging process by improving mitochondrial functions. Mitochondrial dysfunction has been implicated in the pathogenesis of Alzheimer’s disease. By analyzing gene expression in brain of AD patients, they found that the expression levels of BAZ2B and EHMT1 negatively correlate with the expression of key mitochondrial function-related genes, suggesting that BAZ2B and EHMT1 could regulate mitochondrial function in aging human brains.
In this study, the authors have performed a genome-wide RNAi screen and provide the first global view of genes that modulate behavioral aging. They have validated that two conserved epigenetic factors modulate the aging of the nervous system by regulating mitochondrial function. This newly discovered epigenetic regulation of mitochondrial function is critical for achieving healthy aging of the brain. Given the reversible nature of epigenetic regulation, BAZ2B and EHMT1 emerge as promising drug targets against behavioral and cognitive aging.
This work entitled “Two conserved epigenetic regulators prevent healthy ageing” was published online in Nature on February 27, 2020. YUAN Jie, CHANG Siyuan, YIN Shigang, LIU Zhiyang and CHEN Xiu are the first authors with equal contribution. This work was supported by grants to CAI Shiqing (the National Natural Science Foundation of China, 31925022, 91949206 and 81527901; the Strategic Priority Research Program of Chinese Academy of Science, XDBS1020100; Shanghai Municipal Science and Technology Major Project, 2018SHZDZX05; the National Key R&D Program of China, 2018YFC2000400) and grants to JIANG Lubing (National Key R&D Program of China, 2018YFA0507300; National Natural Science Foundation of China, 31771455).
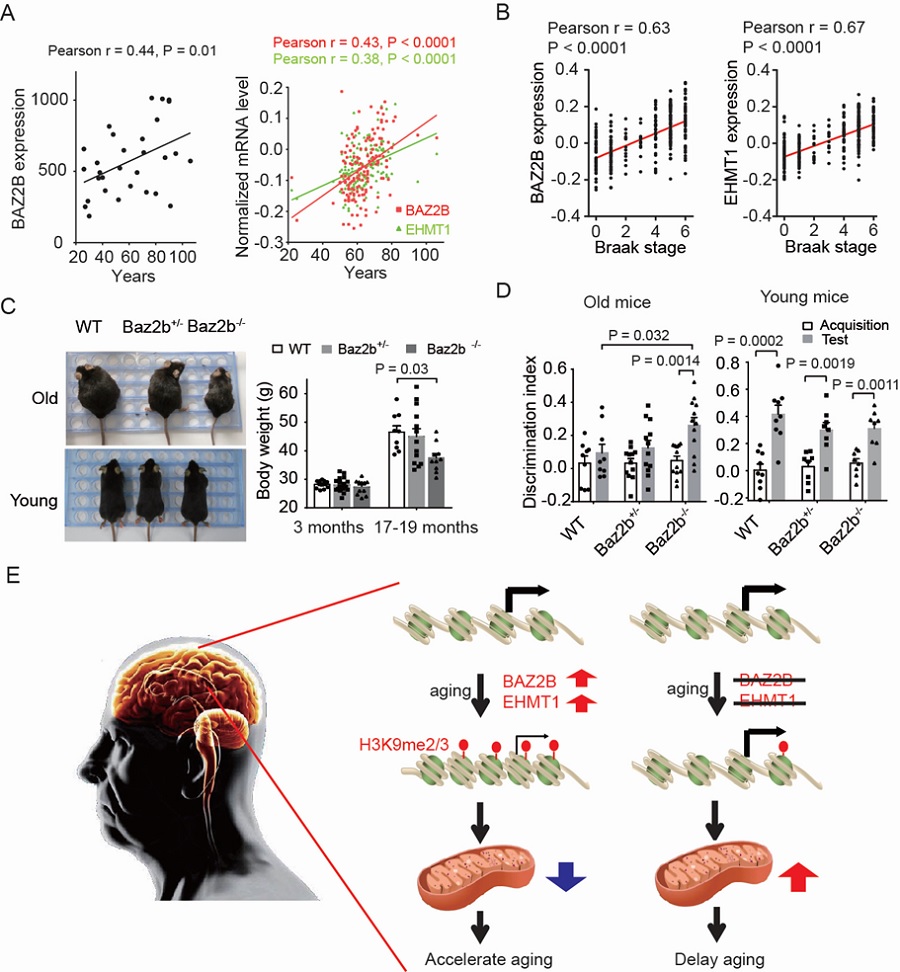
Figure legend: (A) Expression level of BAZ2B and EHMT1 in the prefrontal cortex of human brains at different ages. (B) Expression level of BAZ2B and EHMT1 at different Braak stages in the prefrontal cortex of brains with AD. (C) Body weight of young and old mice. (D) Discrimination index of old and young WT, Baz2b+/- and Baz2b-/- mice. (E) Proposed working model for epigenetic regulation of mitochondrial function and healthy aging.(Image by CEBSIT)
AUTHOR CONTACT:
CAI Shiqing
Institute of Neuroscience and State Key Laboratory of Neuroscience, CAS Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.
E-mail: sqcai@ion.ac.cn
 附件下载:
附件下载: