Time:2021-07-28
A recent study published in Nature revealed the structural basis of antidepressant ketamine action on human NMDA receptors. This work was performed by researchers in Dr. ZHU Shujia’s Lab at the Institute of Neuroscience, State Key Laboratory of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, and Dr. LUO Cheng’s team at the Drug Discovery and Design Center, the Center for Chemical Biology, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences. This work has provided the structural basis of ketamine binding and action on human NMDA receptors, and paved the way for future development of ketamine-based antidepressants.
Major depressive disorder affects about 6-16% of the world population, and even leads to suicide. Antidepressants targeting on monoamine system require prolonged treatment over weeks or months, and are ineffective in one-third of patients. Ketamine, a rapid-acting novel antidepressant, can quickly reduce the core symptoms of depression, notably suicidal ideation within hours after administration, and is still effective in patients with treatment-resistant depression. This discovery is the most important breakthrough in the field of antidepressant. However, ketamine can induce severe psychotomimetic side effects, like dissociative effects. Moreover, ketamine has the abusing potential as recreational drug, which limits its clinical use. Thus, there is increasing scientific and clinical interest in developing rapid-acting antidepressants with fewer side effects.
Previous studies have revealed that ketamine, as a pore blocker of NMDA receptor that is an important glutamate-gated ion channel in the brain, could inhibit the channel activity of NMDA receptor on the postsynaptic membrane to regulate synaptic plasticity and further rescue the stress-induced spine loss in the cortex and hippocampus. Thus, it is important for the development of ketamine-based antidepressants to illustrate the binding site of ketamine in the NMDA receptor and structural basis of ketamine’s action on NMDA receptor.
In this study, the researchers determined the cryo-EM structure of human GluN1-GluN2A NMDA receptor in complex with ketamine, and found the electron density map of ketamine in the transmembrane domain of NMDA receptor. The result confirmed the binding pocket of ketamine was in the central vestibule between the channel gate and selectivity filter. The vestibule is formed by hydrophobic valine (V644 in GluN1 subunits) and leucine (L642 in GluN2A subunits), while the top and bottom of the vestibule are formed by polar threonine and asparagine, respectively. To gain more insights of the interaction between ketamine and the residues around the vestibule, they carried out molecular dynamics simulation to calculate the movement of ketamine in the binding site. The result showed that L642 in GluN2A made the greatest contribution to relative binding energy during ketamine binding through the hydrophobic interaction, while N616 in GluN1 formed hydrogen bond with ketamine among the three asparagine at the bottom. These two amino acids, L642 in GluN2A and N616 in GluN1, were identified as key residues forming hydrophobic interaction and hydrogen bond with ketamine, respectively. In addition, mutations at these two key residues led to the reduced potency of ketamine in blocking the NMDA receptor channel activity.
In conclusion, the authors have uncovered the binding pocket of ketamine in the central vestibule of NMDA receptor, and further validated that hydrophobic interaction of L642 in GluN2A and hydrogen bond formed with N616 in GluN1 are essential to stabilize the binding of ketamine in NMDA receptors. This discovery paved the way for future development of ketamine-based antidepressants.
This work entitled “Structural basis of ketamine action on human NMDA receptors” was published online in Nature on July 28, 2021. ZHANG Youyi, YE Fei and ZHANG Tongtong are the first authors with equal contribution. Dr. ZHU Shujia and Dr. LUO Cheng are corresponding authors. This work was supported by the National Science Foundation of China, Ministry of Science and Technology of the People’s Republic of China, Chinese Academy of Sciences and Shanghai Municipal Government.
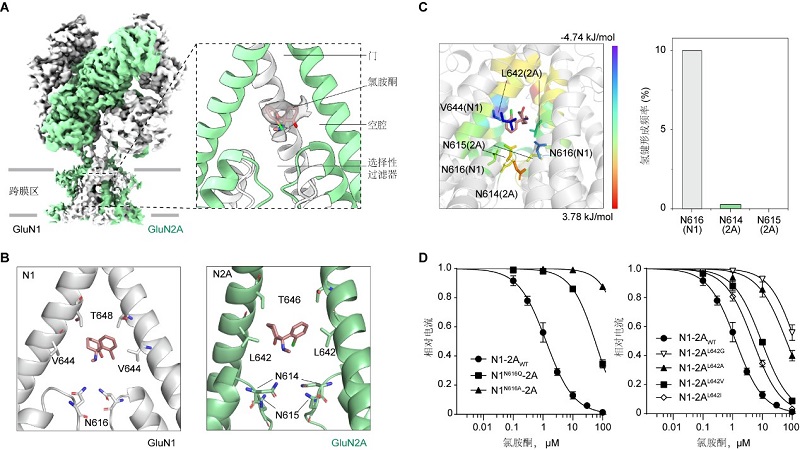
Figure legend: (A) Cryo-EM structures of ketamine bound human GluN1-GluN2A NMDA receptors. (B) Structure model of the binding site of ketamine. (C) Relative binding energy and hydrogen bonding occupancy for residues around ketamine during the molecular dynamics simulation. (D) Ketamine dose-response curves of WT and key residues mutated NMDA receptors. (Image by CEBSIT)

AUTHOR CONTACT:
ZHU Shujia
Center for Excellence in Brain Science and Intelligence Technology , Chinese Academy of Sciences, Shanghai, China.
 附件下载:
附件下载: