Time:2021-06-23
A recent study from Dr. ZHU Shujia’s Laboratory at the Institute of Neuroscience, Center for Excellence in Brain Sciences and Intelligence Technology of the Chinese Academy of Sciences,
This work enriched the study of gating mechanism NMDA receptors, belong to the ionotropic glutamate-gated families, consists of two obligatory GluN1 subunits (bind to glycine) and two GluN2 subunits (2A-2D and bind to glutamate) forming diheteromeric ion channel, play vital role in neuronal development and synaptic plasticity.
These receptors have always been attracted as research interests in the field of neuropharmacology, as their dysfunctions are involved in a large number of neurological disorders, including depressive disorder, epilepsy, schizophrenia, Parkinson's disease and Alzheimer's disease, et al. Meanwhile, various studies showed GluN1-GluN2A receptor preserve abundant pharmacological feature, which let NMDA receptor become the research hot spot.
However, these neurological and psychiatric disorders pose great suffering on both patients and their families. It’s important for us to discover how NMDA receptors function in CNS in turn to understand the pharmacological and structural insight on this receptor.
This study used Cryo-EM to elucidate a gallery of GluN1-GluN2A NMDA receptor structures binds to small molecules constructed at near-atomic resolution around 4 (Figure 1). First of all, the researcher introduced a pair of cysteine crosslinking at inter-dimer interface to lock the receptor in a super-active state. Comparing to previous non-crosslinked structure (PDB:6IRA), they found both GluN1 and GluN2A LBD experiencing inward rolling conformational change, which aligned to former molecular dynamic study.
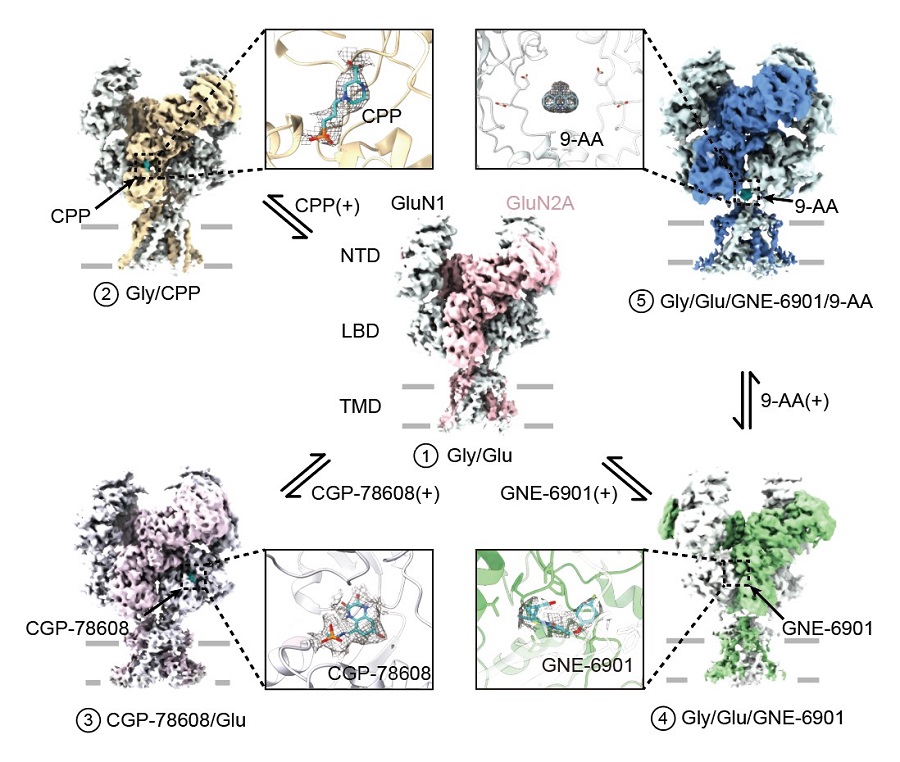
Figure 1 Cryo-EM density of a gallery of human GluN1-GluN2A NMDA receptor
Meanwhile, the researchers use high affinity glutamate binding site (GluN2A) competitive antagonist CPP and glycine binding site (GluN1) competitive antagonist CGP-78608 to mimic an agonist-free inhibited state. After the binding of competitive antagonist to the LBD clamshell, the domain experienced an open conformation compared to agonist bind structure and further close the channel gate. To further achieve an open conformation of the receptor, the researcher used a positive allosteric modulator GNE-6901 to further potentiate the receptor. In previous study, this molecule was found functioned on GluN2A containing NMDA receptor, which can improve EPSC of CA1 pyramidal neuron to enhance brain function. GNE-6901 was found binding at the intra-dimer interface of GluN1 and GluN2A subunits, which separate the dimer interface and further stretch the opening of the channel gate.
Finally, the researcher first visualized a novel modulatory niche on NMDA receptor. The researcher used a foot-in-door open channel blocker 9-Aminoacridine (9-AA) to elucidate the binding site of the small molecule. The Cryo-EM density showed 9-AA reside at the LBD-TMD linker cavity which different from the previous finding of traditional channel blocker. To understand the binding mechanism of 9-AA, the researcher use site-direct mutation and two-electrode voltage-clamp (TEVC) on Xenopus oocytes to confirmed that mutation on “EE-motif” in TM3-D2 linker could largely interfere the binding of 9-AA, suggesting a potential binding site of NMDA receptor channel blocker. This study enriched the research of NMDA receptor structure and functional study, unraveled gating mechanism and provide important structural evidence for drug design, screening and development (Figure 2).
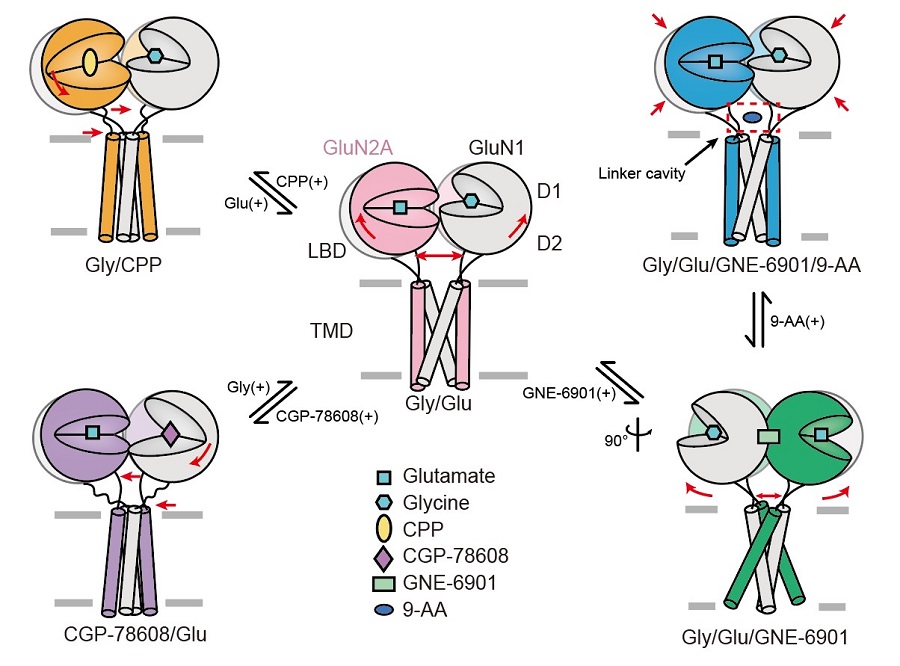
Figure 2 Gating transition of human GluN1-GluN2A NMDA receptor
This work entitled “Gating mechanism and a modulatory niche of human GluN1-GluN2A NMDA receptors” was published online in Neuron on the 28th, June. The Ph. D student WANG Han from Dr. ZHU Shujia’s lab was the first author of the study. Graduate student LV Shiyun and HUANG Xuejin participate in this project. Also, with effort from ZHANG Jinbao, PAN Yijie. Great effort on Cryo-EM data collection from Center for Excellence in Brain Science and Intelligence Technology, Zhejiang University, Institute of Biophysics and Shanghaitech University. This work also benefited greatly from the kind help from the Institute de Biologie de I’Ecole Normale Supérieure (IBENS).
This study was supported by grants from National Nature Science Foundation of China, Ministry of Science and Technology of the People′s Republic of China, Chinese Academy of Science, Science and Technology Commission of Shanghai Municipality and the European Research Council.

AUTHOR CONTACT
ZHU Shujia
Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.
E-mail: shujiazhu@ion.ac.cn
 附件下载:
附件下载: