Time:2025-01-08
A recent study published in Nature Aging on January 8, 2025, by researchers from the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, reveals that neuroprotective and neurotoxic astrocytes represent transitional states rather than distinct subtypes, as shown through time-series multiomic sequencing. Neuroprotective astrocytes emerge as an intermediate state during the transition from a nonreactive to a neurotoxic state in response to neuroinflammation, a process regulated by the mTOR signaling pathway. In Alzheimer's disease (AD) and aging, an imbalance between neurotoxic and neuroprotective astrocytes was observed in animal models and human patients. Furthermore, targeting mTOR in astrocytes using rapamycin or shRNA reduced neurotoxic astrocyte activity in neurodegenerative mouse models. These findings uncover a mechanism by which astrocytes initially exhibit neuroprotective functions before transitioning to a neurotoxic state under neuroinflammatory conditions, and they highlight mTOR modulation in astrocytes as a potential therapeutic approach for neurodegenerative diseases.
Astrocytes, a type of glial cell, are known for their supportive and protective functions in the nervous system. However, under disease conditions, astrocytes transition to reactive states with neuroprotective or neurotoxic characteristics. Previous studies have suggested that astrocytes in different disease contexts exhibit distinct reactive states, such as neuroprotective states in ischemic stroke models and neurotoxic states induced by inflammatory microglia via interleukin-1α (IL-1α), tumor necrosis factor (TNF), and complement component C1q. However, these states were primarily identified based on transcriptomic analyses at specific time points, leaving their dynamic transitions unclear.
To explore the state transition process of astrocytes under neuroinflammatory conditions, the researchers treated primary cultured mouse astrocytes with IL-1α, TNF, and C1q (ITC) and conducted time-series transcriptomic, proteomic, and single-cell sequencing analyses. They discovered that astrocytes do not transition directly from a resting state to a neurotoxic state. Instead, they pass through an intermediate neuroprotective substate (Figure 1). mTOR signaling was identified as a critical regulator of this state transition.
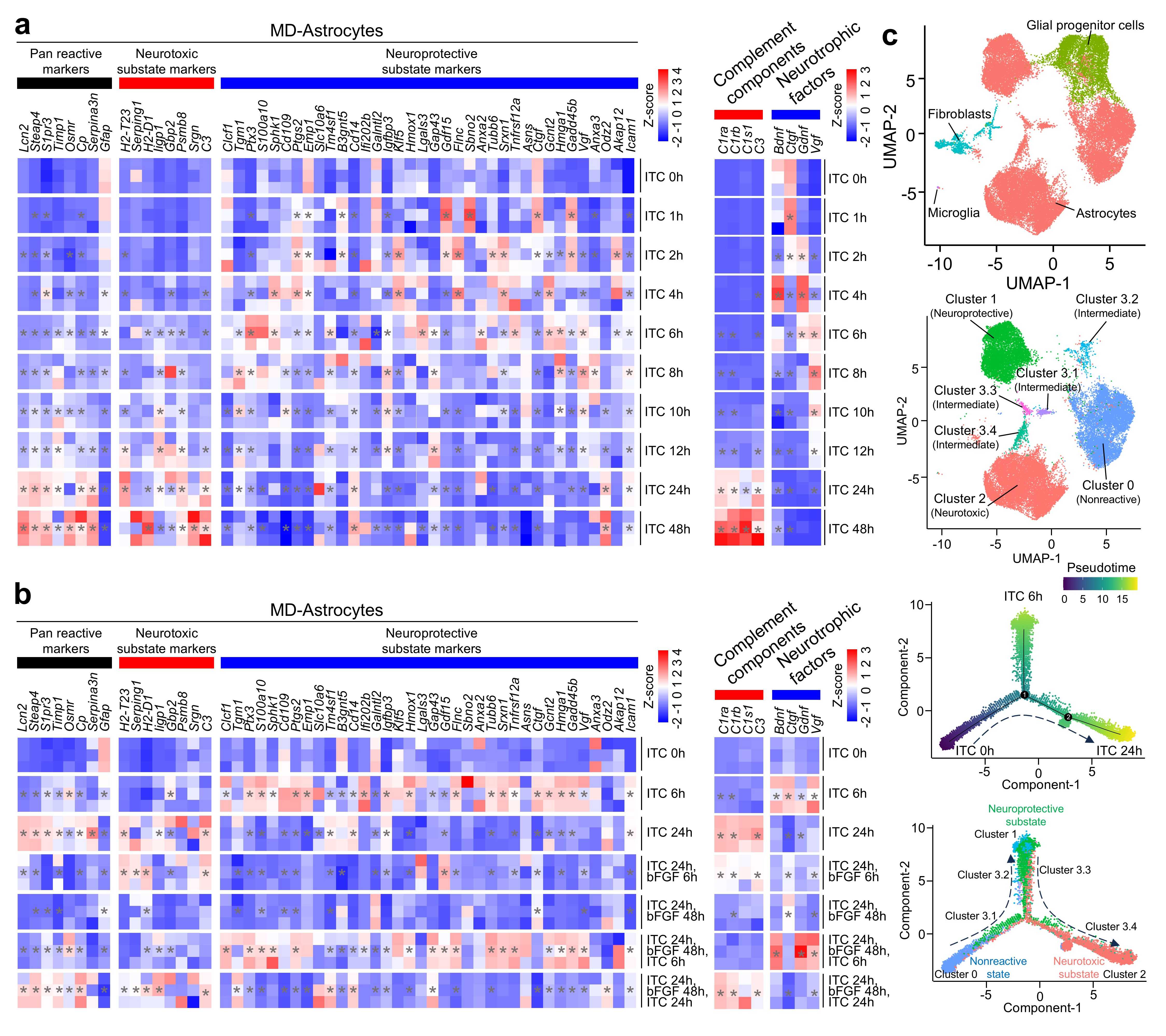
Figure 1. Astrocyte substate transitions under ITC stimulation. (a) Heatmap showing marker gene expression profiles of astrocyte substates over time. (b) Expression profiles of astrocyte substate markers under activation-deactivation-reactivation conditions. (c) Single-cell trajectory analysis illustrating astrocyte state transitions.
Further analysis of single-cell sequencing data from 5XFAD mice (a model for AD), AD patients, and aging individuals revealed a progressive decrease in neuroprotective astrocytes and an increase in neurotoxic astrocytes during AD progression and normal aging. This imbalance may contribute to persistent neuroinflammation and cognitive decline in these conditions.
Finally, the researchers tested the therapeutic potential of targeting mTOR in astrocytes in LPS-induced PD-like mice and 5XFAD mice. Treatment with the mTOR inhibitor rapamycin suppressed neurotoxic astrocyte activation, reduced dopaminergic neuron death in the PD model, and mitigated hippocampal neuronal degeneration in the AD model. Additionally, astrocyte-specific knockdown of mTOR using an AAV-delivered shRNA system achieved similar protective effects (Figure 2).
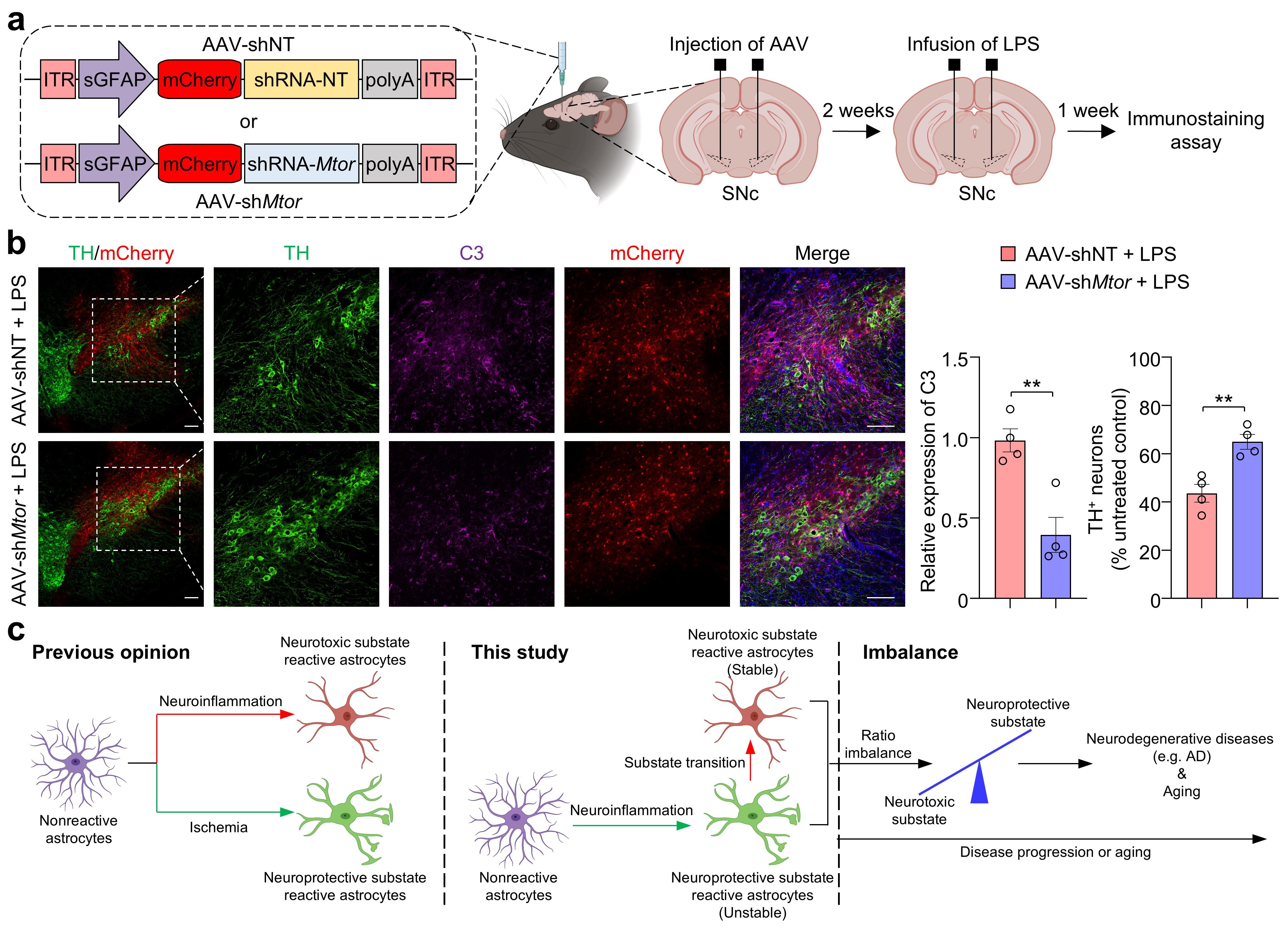
Figure 2. Astrocyte-specific mTOR knockdown alleviates neurodegeneration in a PD-like mouse model. (a) Experimental workflow for AAV-mediated astrocyte-specific mTOR knockdown. (b) Reduced activation of neurotoxic astrocytes and decreased dopaminergic neuron death in treated mice. (c) Schematic representation of the hypothesis of state transition observed in reactive astrocytes.
This study provides experimental evidence for the dynamic substate transitions of astrocytes under neuroinflammatory conditions and highlights the critical role of mTOR signaling in this process. The findings suggest that precise modulation of astrocyte substate transitions could be a promising therapeutic strategy for treating neurodegenerative diseases such as AD and PD.
The study, titled "Modulating mTOR-dependent astrocyte substate transitions to alleviate neurodegeneration," was led by Dr. ZHOU Haibo’s research group at the Institute of Neuroscience. Drs. ZHANG Liansheng and XU Zhengzheng, and graduate students JIA Zhiheng and CAI Shicheng are co-first authors, while Dr. ZHOU Haibo and Dr. ZHANG Liansheng are corresponding authors. This research was supported by the Ministry of Science and Technology, Chinese Academy of Sciences, Shanghai Municipal Science and Technology Commission, and the Shanghai Brain-Intelligence Project.
https://www.nature.com/articles/s43587-024-00792-z
Keywords: Astrocyte substates, mTOR signaling, Alzheimer’s disease, Parkinson’s disease, neurodegeneration
AUTHOR CONTACT:
ZHOU Haibo
Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China
Phone: 86.21.5492.1706
Email: hbzhou@ion.ac.cn
 附件下载:
附件下载: