Time:2024-01-05
On January 4, 2024, the research paper titled "Piezo1-dependent regulation of pericyte proliferation by blood flow during brain vascular development" was published online in the journal "Cell Reports." This study was conducted by DU Jiulin’s research group at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences. In this work, the researchers created a zebrafish model for in vivo labeling of brain pericytes, and systematically explored the developmental dynamics of brain pericytes during the early embryonic stage, revealing the promoting effect of blood flow on the proliferation of pericytes after ingress into the brain. They further elucidated that this process relies on the activation of the mechanosensitive ion channel Piezo1 in vascular endothelial cells (ECs) and its downstream Notch signaling.
Brain function relies on a complex and effective vascular network that provides necessary nutrients and removes waste products. To maintain the homeostasis of neural tissues and normal neural activity, the brain vasculature forms the blood-brain barrier (BBB) during development, strictly controlling substance exchange between blood and brain parenchyma. Abnormalities in the blood-brain barrier are closely associated with various brain diseases, such as Alzheimer's disease. Pericytes, as mural cells tightly attached to the outer side of EC tubes in capillaries, play a crucial role in maintaining the integrity of BBB. Therefore, understanding the developmental process of brain pericytes is essential for studying the formation and maintenance of BBB. Previous research has indicated that blood flow, the most important functional behavior of blood vessels, participates in regulating the development of brain ECs. However, whether blood flow affects the growth of brain pericytes remained an unexplored question.
To observe the dynamic development of brain pericytes, researchers employed zebrafish as a vertebrate model and established an in vivo model with specifically marked pericytes using CRISPR/Cas9 gene-editing techniques. The results of long-term time-lapse imaging revealed that brain pericytes originate early from the precursor cells on pericerebral blood vessels, and they expand their population majorly by proliferation after ingress into the brain. By pharmacologically altering blood flow velocities, researchers found that blood flow up-regulates the pericyte coverage of brain vessels, which is mainly achieved through promoting the proliferation of brain pericytes.
Furthermore, researchers discovered that the mechanosensitive ion channel Piezo1, expressed on ECs, senses the changes of blood flow and mediates the effect of blood flow on pericyte proliferation.
How does the influence of blood flow transmit from ECs to pericytes? Researchers observed that the activation of Piezo1 significantly increased the activity of Notch signaling in ECs. Specific enhancement or inhibition of Notch signaling in ECs resulted in corresponding up-regulation or down-regulation of the proliferation frequency of brain pericytes. Increasing blood flow or Piezo1 activity failed to cause a significant change in the pericyte coverage of brain vessels when EC Notch signaling was suppressed. This results suggest that EC-intrinsic Notch signaling mediates the regulation of blood flow in the development of brain pericytes, as the downstream of Piezo1. Additionally, by specifically enhancing or inhibiting the outward transmission of Notch signaling in ECs, researchers provided evidence that the ECs with enhanced Notch signaling directly activated Notch signaling in pericytes promoted their division.
This study uncovers a novel regulatory mechanism for blood flow regulation of brain vascular development, and also provides new perspectives for understanding brain pericyte development. For researchers actively seeking methods to treat neurological disorders, these findings may offer new therapeutic strategies. For example, up-regulating the activity of Piezo1 or the intensity of Notch signaling in ECs promotes the proliferation of brain pericytes, thereby improves the function of brain vessels, facilitates the recovery of brain function in patients with diseases such as Alzheimer's, vascular dementia, and stroke.
Investigator DU Jiulin and associate investigator LI Jia are the corresponding authors of this paper. Dr. ZI Huaxing, a postdoctoral researcher at DU Jiulin’s lab, is the first author. Assistant investigator Dr. PENG Xiaolan participated in the live imaging of zebrafish. The graduate student XIE Tianyi, Dr. LIU Tingting, Dr. LI Hongyu, and the Lab Manager BU Jiwen made important contributions to the production of crucial zebrafish lines involved in this project. In addition, CAO Jianbin from the Taizhou Hospital of Zhejiang Province affiliated to Wenzhou Medical University, participated in the pharmacological experiments. The optical platform of the Center for Excellence in Brain Science and Intelligence Technology provided crucial technical support. This research is supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Shanghai Natural Science Foundation, the SA-SIBS Scholarship Program, and the Youth Innovation Promotion Association of Chinese Academy of Science.
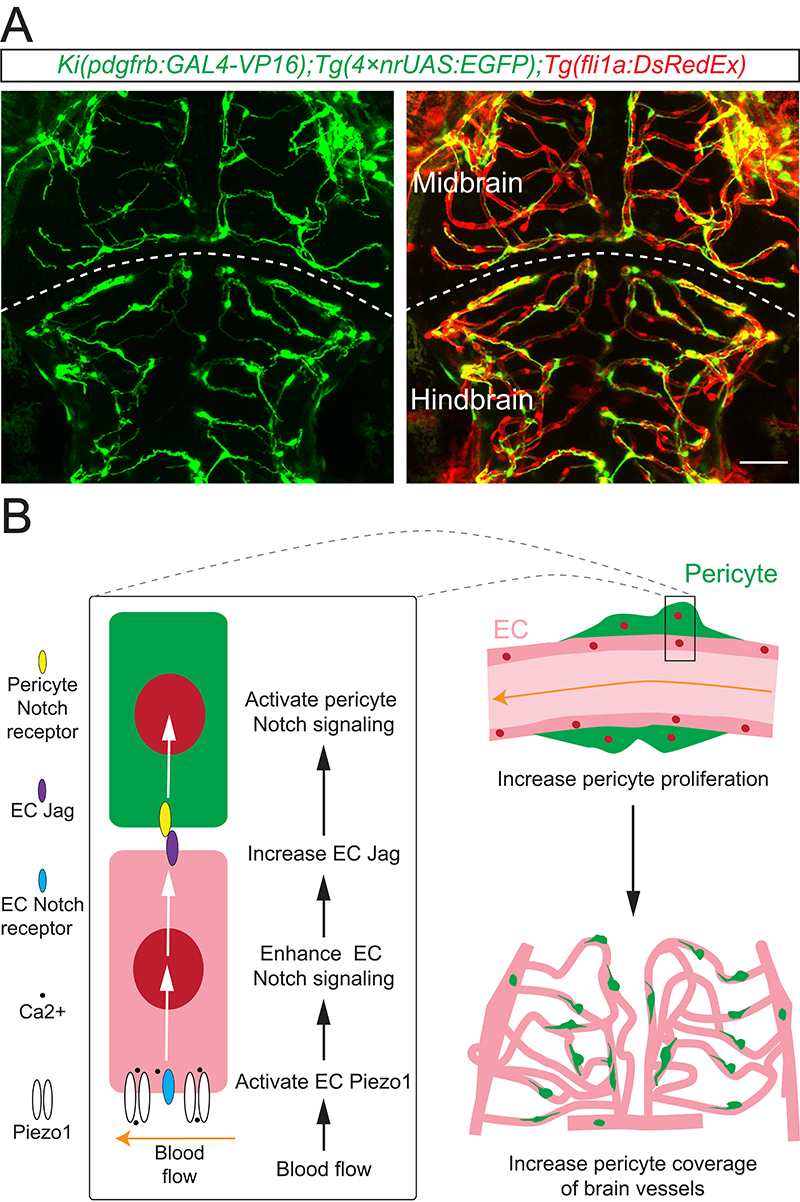
A. Example showing the imaging of brain vessels and pericytes in zebrafish larvae. EC in red, pericyte in green. B. Schematic diagram illustrating the mechanism of the regulation of brain pericyte development by blood flow. Blood flow activates Piezo1 in ECs, leading to an up-regulation of Notch signaling in ECs. This, in turn, promotes the expression of Notch ligands (Jags) in ECs, facilitating the activation of Notch signaling in pericytes. Ultimately, this cascade leads to an increase in the proliferation of brain pericytes and their coverage on brain vessels.
 附件下载:
附件下载: