Time:2023-10-28
Dr. LI Xue’s lab from the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, developed a hyperflexible electrode array for long-term recording and decoding of intraspinal neural activities. This work was recently published on Advanced Science.
This study utilized substrate-free multi-layer design and advanced nanofabrication techniques to manufacture a hyper-flexible, multi-channel spinal cord interface, which implanted in the spinal cord ventral horn of mice for up to one year of signal recording. This device is the first time reported hyper-flexible spinal cord neural interface, which fills the gap in methods for long-term stable single-cell intraspinal recording. It brings new potentials for clinical monitoring and intervention of neural system diseases such as spinal cord injuries, Parkinson's, ALS, and other movement disorders.
The spinal cord is a critical central for the communication between the brain and peripheral nerves. However, the nature of the delicate spinal cord neurons and the constant motion of the spinal cord make spinal cord recording extremely challenging; and there was no solution for long-term stable, high channel-count, single-cell resolution, and high bandwidth spinal cord neural interface. Since long-term stable recording of spinal cord neurons can be an important research method and is highly promising for diagnosing and treating of various motor and sensory disorders. Therefore, it is urgent to develop such spinal recording technologies.
There are two kinds of traditional spinal cord interfaces: one is epidural electrodes that mainly record field potential signals, and the other is intraspinal electrodes that can record single-cell action potentials within the spinal cord. Compared to epidural electrodes, intraspinal electrodes offer superior spatial resolution that enables more precise motor signal extraction, complex motor decoding, and fine motor modulation with the help of external devices. However, traditional intraspinal rigid electrodes often induce strong immune responses due to significant mechanical mismatch with spinal cord tissue, which results in spinal cord scar formation near the interface and ultimately fails in long-term recording and modulation. Additionally, the spinal cord's movement can disform the electrodes causing device breakage, signal failure, and secondary tissue damage.
To overcome these challenges, Dr. Xue Li's team used nano-techniques to design and fabricate spinal cord neural interfaces to significantly reduce the interface's thickness, which effectively addressed the mechanical mismatch between the interface and the spinal cord tissue. They also used biocompatible polymer materials like SU-8 and polyimide to substantially enhancing the electrode's fatigue and aging resistance (Fig. a). Through one-year recording of spinal cord ventral horn signals, they verified the recording stability (Fig. b-d) of the hyper-flexible spinal cord interface. The ultra-thin structure also enabled the interface to bend along with the cell movements, greatly improving its tissue compatibility. Triple immunofluorescence imaging showed there was minimal inflammation around the interface and stable neuron density (Fig. e).
To further prove the recording effectiveness of the spinal cord interface, neural signals from mouse spinal cord during wheel running tasks were recorded and synchronized with movements of multiple hindlimb joints (Fig. f). The spinal cord spikes and multi-band LFPs achieved precise decoding of hindlimb kinematics using LSTM model (Fig. g). For example, decoding of foot movement based on action potential signals achieved an R2 of 0.95 (Fig. h). Further neural trajectory analysis revealed distinct neuronal dynamic patterns in PCA subspaces during movement for both single-cell and different-band LFPs (Fig. i).
This work successfully addresses the gap in tools for long-term stable single-cell recording within the spinal cord. It introduces the development of ultra-flexible spinal cord neural interfaces and demonstrates their high throughput, high bandwidth, excellent biocompatibility, and robustness in the spinal cord ventral horn. This achievement provides a powerful tool for fundamental research on spinal cord neural circuits and opens new avenues for clinical intervention methods in neural system diseases related to spinal cord injuries, Parkinson's, ALS, and other movement disorders.
This work was supervised by Dr. LI Xue, and conducted by post-docoral FAN Jei, LI Xiaocheng, and research assistant WANG Peiyu with the contribution of all team members and collaboration with Dr. ZHAO Zhengtuo from the Center for Excellence in Brain Science and Intelligence Technology. This study was supported by Chinese Academy of Sciences, Shanghai Municipal People's Government, Ministry of Science and Technology, and the National Natural Science Foundation of China.
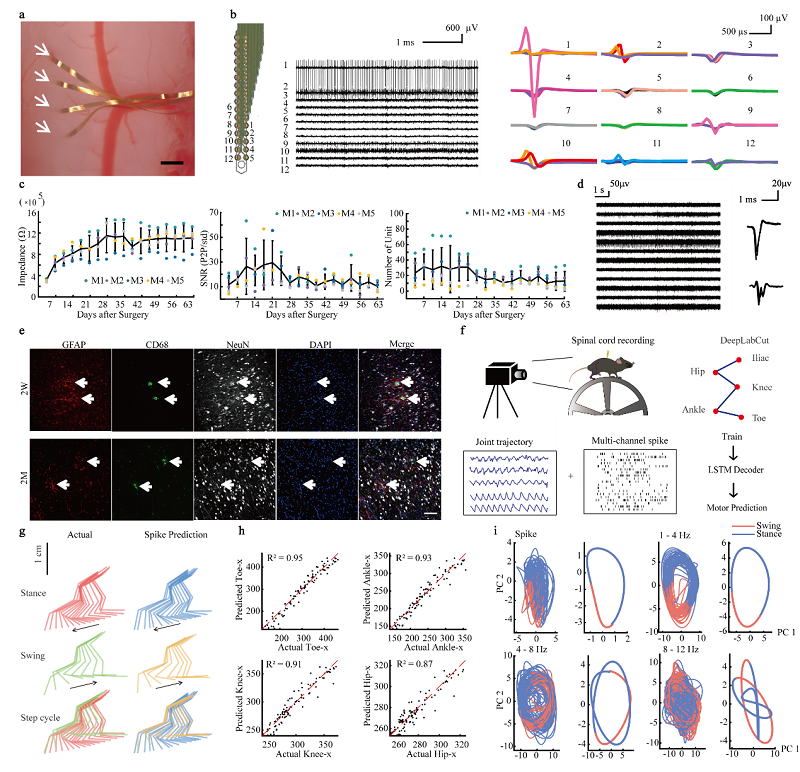
Figure Legends: (a). Implantation of the hyper-flexible spinal cord interface. (b). Acute neural recording after implantation. (c). Signal tracking within 9 weeks after electrode implantation. (d). Recording result one-year after electrode implantation (e). Immune responses induced by the implantation of the electrode (f). Schematic for the mice behavioral paradigm and spinal cord neural signal decoding process (g). Decoding of hindlimb movement trajectory decoded using intraspinal signals (h). Accuracy of decoding using spinal cord neural signals (i). Neural trajectories of population spinal cord signals during movement after dimension reduction
 附件下载:
附件下载: