Time:2023-09-26
A recent study published in Nature Communications revealed the topographic organization of high-level visual areas in human and monkey brains. This work was performed by researchers in Dr. CHANG Le’s Lab at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences. This work systematically investigated how visual features are represented and organized in the primate temporal lobe by combining functional MRI, electrophysiology, and deep learning, paving the way for the development of artificial visual systems with brain-like topographic organization.
The inferotemporal cortex (ITC) supports our supreme object recognition ability. A series of studies carried out in primate brains have revealed multiple ITC subregions specialized for specific object categories or features. For example, face-processing subregions have been identified in both human and monkey brains. However, some important questions remain unanswered, for example: 1) the brain regions identified with specialized functions cover only about half of the monkey ITC, and it’s unclear what the rest does; 2) most studies have been conducted in a single species, either human or a non-human primate species, and a detailed comparison of the ITC’s topographic organization between human and monkey brains is still needed.
To answer these questions, we need to map out the neural selectivity across the ITC in both species with a rich set of visual features and perform quantitative analyses on the obtained maps. As demonstrated in previous studies, the responses of ITC neurons can be accurately predicted by linear combinations of unit activities in convolutional neural networks. Passing 200k natural images through a convolutional neural network and performing independent component analysis (ICA) on the responses of its deep layer units resulted in 25 independent components (Figure 1a). To map out the neural selectivity to these 25 features in the primate brain, functional MRI experiments were performed on macaque monkeys and human subjects while they fixated on the images (Figure 1b).
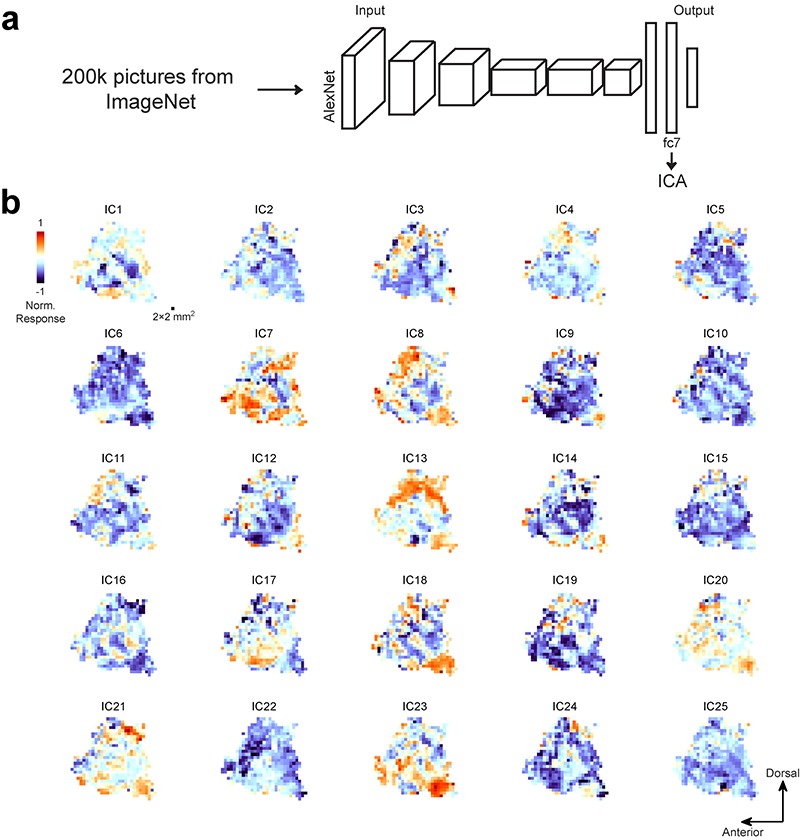
Figure 1. a) 200k natural images from ImageNet were fed into AlexNet, a neural network for object recognition. Independent component analysis was performed on the responses of units in the penultimate layer (fc7). 25 independent components (ICs) were extracted to build an object space. b) 25 feature maps of the left temporal lobe for one monkey are plotted on the cortical flat, one map for each IC.
The experimentally measured neural selectivity of each brain location was compared to a collection of interpretable visual features, including those investigated in previous studies (Figure 2a). This allowed us to quantitatively link our feature maps to already known features and identify novel features represented in the temporal lobe. A new brain region encoding a novel feature was localized, and its selectivity was confirmed by electrophysiology (Figure 2b-e). These feature maps also allowed quantitative analyses of the topographic organization of the temporal lobe, demonstrating the existence of a pair of orthogonal gradients that differ in spatial scale in the monkey brain. This intricate structure of functional gradients in the monkey data did not appear in a new type of neural network model recently proposed to account for ITC’s topographic organization, providing important constraints for the development of relevant computational models. Furthermore, two previously unknown differences between the two species were identified: 1) the human brain shows a stronger preference for inanimate objects than the monkey brain; 2) the topographic organization of the monkey brain is more regular than that of the human brain (Figure 2f-h). These two differences are likely due to our need to recognize various types of artificial objects and symbols, which may expand the cortical areas responsible for inanimate objects and distort the originally ordered topographic organization in the monkey brain during evolution.
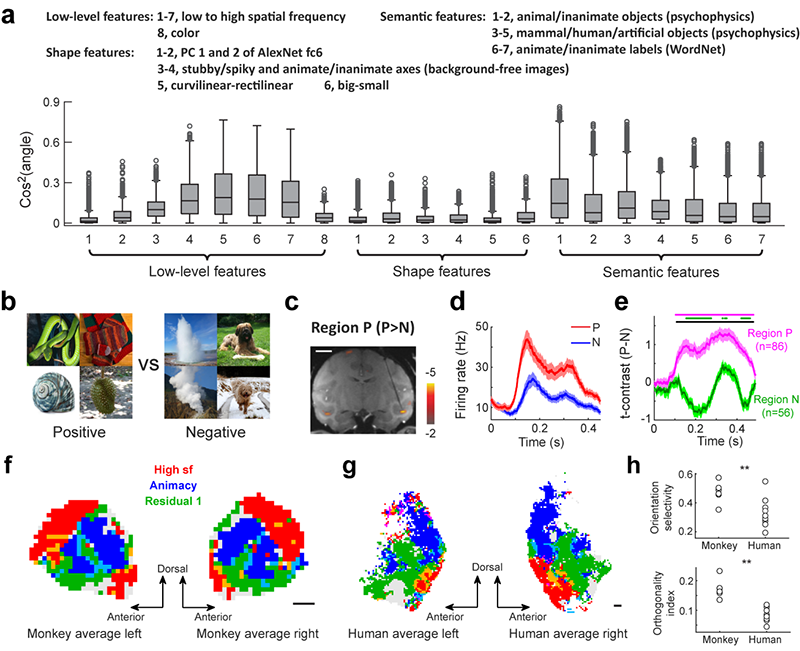
Figure 2. a) Similarity between 25D neural features of three monkeys and 21 interpretable visual features. b) Preferred (positive) and non-preferred (negative) images for a previously unknown subregion in the monkey’s ITC. c) A coronal slice showing the location of a positive-group preferred region targeted for electrophysiology. d) Responses of an example neuron to images in the positive and negative groups. e) Average time courses of t-contrasts between the two conditions, with purple and green lines indicating positive- and negative-group preferred regions, respectively. f) Within a single monkey feature map, different colors are assigned to locations with different preferences: blue, red, and green represent preferences for three important features (high sf, animacy, and residual feature 1). g) Same as (f), but for human subjects. H) Comparison of the topographic organization of monkey and human temporal lobes using two indices: orientation selectivity of feature gradients (top) and orthogonality between different gradients (bottom). Both indices indicate a more regular organization in the monkey temporal lobe.
This study has systematically characterized high-dimensional visual feature maps in the temporal lobe of two primate species, paving the way for more in-depth research into the high-level visual areas. While previous studies have highlighted highly quantitative analyses of the feature selectivity of ITC neurons using sophisticated computational models, the characterization of the topographic organization has remained largely qualitative. By addressing this issue in aquantitative manner, this study has revealed clear differences in the topographic organization of high-level visual areas between different species and different systems, shedding light on the development and evolution of the primate inferotemporal cortex.
This paper entitled “High-dimensional topographic organization of visual features in the primate temporal lobe” was published in Nature Communications on September 22, 2023. YAO Mengna, WEN Bincheng, YANG Mingpo, and GUO Jiebin are the co-first authors of this paper. This work was supported by grants from the Ministry of Science and Technology, Chinese Academy of Sciences, Shanghai Municipality, and National Natural Science Foundation of China.
 附件下载:
附件下载: