Time:2023-09-08
A recent study published in Nature Metabolism demonstrated that astrocyte-specific overexpression of TMEM164 alleviating symptoms of neurodegenerative diseases in mouse models. This work was performed by the researchers in Dr. ZHOU Haibo’s Lab at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, State Key Laboratory of Neuroscience. This work has successfully identified transmembrane protein TMEM164 as a key regulator of neurotoxic astrocyte reactivity, and found that TMEM164 overexpression inhibited the induction of neurotoxic reactive astrocytes and neuronal death in both mouse and human astrocytes, as well as in Alzheimer’s disease (AD) and Parkinson’s disease (PD) mouse models. This discovery provides a potential therapeutic target for neurodegenerative diseases associated with neurotoxic reactive astrocytes.
Under conditions of brain injury or neurodegenerative diseases, astrocytes undergo distinct reactive states. Genetically profiling reactive astrocytes from inflammatory and ischemia mouse models revealed the induction of two different subtypes of reactive astrocytes, namely neurotoxic and neuroprotective astrocytes. Cytokines interleukin-1 alpha (IL-1α), tumor necrosis factor (TNF), and complement component subunit 1q (C1q) released from classically activated neuroinflammatory microglia have been reported to be sufficient and necessary to induce neurotoxic reactivity of astrocytes in vitro. Neurotoxic reactive astrocytes lose their normal functions, including the support of neuronal survival and outgrowth, synaptogenesis, and phagocytosis. Instead, they acquire detrimental effects such as inducing the death of neurons through saturated lipids.
Previous studies have shown that abundant neurotoxic reactive astrocytes are present in brain samples from patients with AD, PD, Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS). This suggests that the induction of neurotoxic reactive astrocytes is strongly associated with the pathogenic progression of various diseases in human patients. Although transcriptome signatures of distinct reactive astrocyte subtypes and the possible mechanisms of astrocyte neurotoxicity have been elucidated previously, the key regulators that modulate neurotoxic astrocyte reactivity remain unknown.
To identify key targets for regulating the activation of neurotoxic reactive astrocytes, researchers integrated and analyzed transcriptomic sequencing data from activated, deactivated, and reactivated neurotoxic reactive astrocytes, as well as single-nucleus sequencing data from the brains of patients with various neurodegenerative diseases. Through this analysis, they identified the transmembrane protein TMEM164 as an early response factor during the activation of neurotoxic reactive astrocytes, which may be closely associated with the development of multiple neurodegenerative diseases. Overexpression of TMEM164 in cultured mouse and human astrocytes inhibited the activation of neurotoxic reactive astrocytes, maintained their normal functions of phagocytosis (Figure 1A-B) and synaptogenesis (Figure 1C-D), and suppressed neurotoxic astrocyte-induced neuronal death, demonstrating the neuroprotective role of TMEM164 (Figure 1E-F).
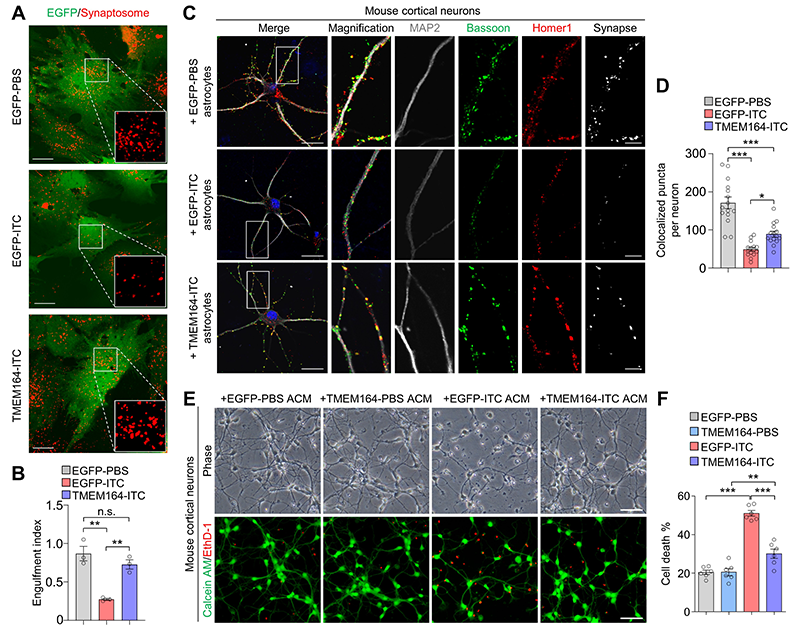
Figure 1. Transmembrane protein TMEM164 regulates the activation of neurotoxic reactive astrocytes. (A-B) TMEM164 overexpression maintains normal astrocyte functions of phagocytosis. (C-D) TMEM164 overexpression maintains normal astrocyte functions of synaptogenesis. (E-F) Overexpression of TMEM164 suppresses neuronal death induced by neurotoxic reactive astrocyte-conditioned medium.
To further demonstrate the neuroprotective effects of TMEM164 overexpression in astrocytes in vivo, researchers developed an adeno-associated virus (AAV) therapeutic strategy that efficiently and specifically inhibits the activation of neurotoxic reactive astrocytes in specific brain regions or even throughout the whole brain. This strategy successfully prevented the activation of neurotoxic reactive astrocytes and neuronal death in AD and PD mouse models (Figure 2).
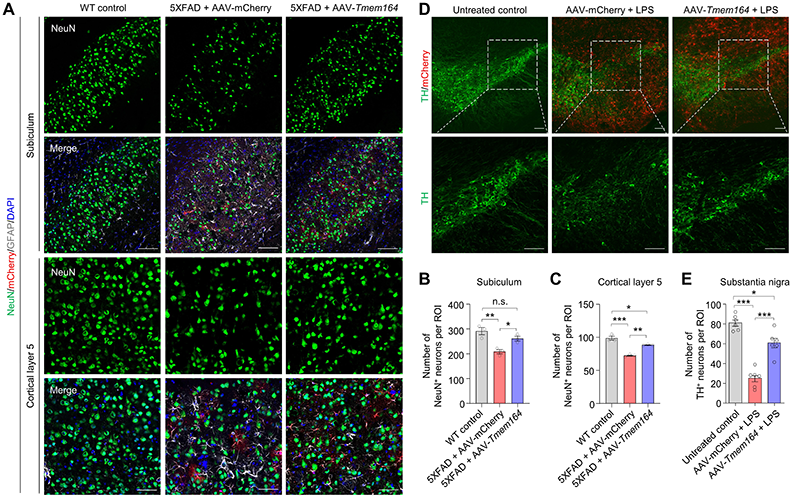
Figure 2. AAV-mediated astrocyte-specific overexpression of TMEM164 inhibits neuronal death in AD and PD mouse models. (A-C) AAV-mediated astrocyte-specific overexpression of TMEM164 throughout the brain inhibits neuronal death in the subiculum and cortical layer 5 of 5XFAD mice. (D-E) AAV-mediated astrocyte-specific overexpression of TMEM164 inhibits neuronal death in the substantia nigra in an LPS-induced PD mouse model.
This study provides the first evidence of the regulatory role of the transmembrane protein TMEM164 in astrocyte activation within the nervous system. Furthermore, the development of an AAV-mediated strategy for astrocyte-specific overexpression of TMEM164 offers a novel therapeutic target and approach for treating neurodegenerative diseases, including Alzheimer's disease and Parkinson's disease, providing new possibilities for clinical interventions in various neurodegenerative disorders.
This work entitled “Alleviating symptoms of neurodegenerative disorders by astrocyte-specific overexpression of TMEM164 in mice” was published online in Nature Metabolism on September 7, 2023. ZHANG Liansheng, JIA Zhiheng, and WU Qiang are the first authors with equal contribution. ZHOU Haibo and XU Zhengzheng are the correspondence authors. This study was supported by the Ministry of Science and Technology, Chinese Academy of Sciences, Shanghai Municipal Science and Technology Commission, and Shanghai Institute for Brain-Inspired Intelligence.
Keywords: Neurotoxic reactive astrocytes, TMEM164, Neurogenerative diseases
AUTHOR CONTACT:
ZHOU Haibo
Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.
Phone: 86.21.5492.1706; Email: hbzhou@ion.ac.cn
 附件下载:
附件下载: