Time:2022-06-02
A paper published recently in Current Biology demonstrated a unique design of neural microcircuit in the ventromedial hypothalamus (VMH). This work was performed by researchers in Dr. XU Huatai’s lab at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, and the State Key Laboratory of Neuroscience, Shanghai Center for Brain Science and Brain-Inspired Intelligence Technology. This study applied combinatory approaches to reveal a distinct development transition of neural microcircuit in the VMH, which featured dense electrical coupling at the early developmental stage and sparse chemical synapses but prominent neuropeptide transmission in the adult (Figure 1). The absence of chemical synapse was further observed in many other hypothalamic nuclei. These findings provide a solid microcircuit basis for a better understanding of hypothalamic functions.
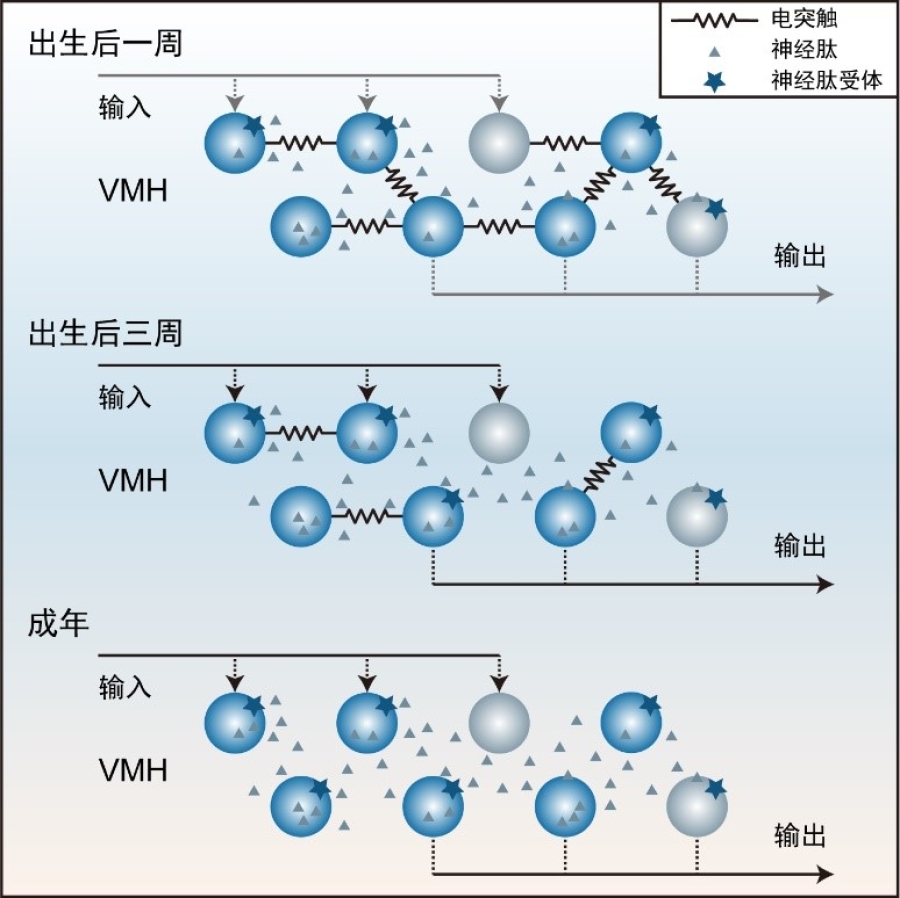
Figure 1: Summary of microcircuits in the VMH. After birth till adulthood in the VMH, the coupling frequency of electrical synapses decreased while the effect of neuropeptide modulation increased.
Neuroscience has evolved a lot from the cellular level to the system level. While long-range projections bridge different brain regions to coordinate behaviors, neural microcircuits assist in integrating information from various inputs and sending out signals after processing. Therefore, it is essential to dissect neural circuits at both levels to understand the neural basis of behaviors.
The hypothalamus is a multi-nuclei structure, and inter-nucleus connections have been extensively investigated. However, the microcircuit within most hypothalamic nuclei remains less examined. To fill this gap, researchers applied quadruple whole-cell patch-clamp recordings on hypothalamic slices. They firstly found a high frequency of electrical coupling between neurons during the early development of the VMH, which decreased gradually afterward (Fig. 2A). This electrical synapse was mediated by connexin 36 (Cx36) as conditionally knockout of Cx36 in the VMH significantly reduced the electrical coupling. It has been reported in the neocortex that electrical synapses were the blueprint of chemical synapses. However, from early development to adult, chemical synapses were seldom detected by paired recordings in the VMH (Fig. 2B), indicating sparse intrinsic synaptic connectivity in the adult VMH. Similarly, in other hypothalamic areas like the arcuate nucleus (ARC), the dorsomedial hypothalamus (DMH), the lateral hypothalamic nucleus (LHA), the premammillary nucleus (PM), the paraventricular hypothalamus (PVH), and the suprachiasmatic nucleus (SCN), paired recordings only detected a few electrical but no chemical synapses. These observations reveal the sparse chemical connectivity as a generalized feature in the hypothalamic microcircuit.
Paired recordings efficiently detect synaptic connections within a limited range (<200 micrometers). To consolidate the sparse connectivity in a broader scope, researchers applied ChR2-assisted circuit mapping (CRACM) in the adult VMH (Fig. 2C). They found when even one-third of neurons in the range of ~400 micrometers were activated, the peak amplitudes of optogenetic excitatory postsynaptic currents (EPSCs) were insignificant to the spontaneous ESPCs of responsive neurons (Fig. 2D). This result indicated probably very few activated neurons in a much broader range with synaptic connections to the recorded cells. Besides, the modified rabies virus-based retrograde monosynaptic tracing result further supports the scarceness of chemical synapses in the VMH (Fig. 2E).
Hypothalamus is enriched with neuropeptides. To explore the potential role of neuropeptides in neuronal communication in the VMH, researchers incubated VMH slices with agonists and found that an analog of dynorphin could hyperpolarize VMH neurons. Consistently, VMH neurons showed hyperpolarization after a short period of high-frequency stimulation of adjacent neurons, which can be blocked by the antagonist of dynorphin receptor KOR (kappa opioid receptor) (Fig. 2F), suggesting an inhibitory role of the neuropeptide dynorphin in local neuronal modulation. The expression of dynorphin and KOR increased during the developmental period. Consistently, the hyperpolarization effect of the KOR agonist on VMH neurons was much weaker in pups, revealing the more effective role of neuropeptide for local neuronal communication in the VMH during development.
Conclusively, this work revealed the microcircuit within VMH from a development perspective. It strongly suggests that the sparse chemical connectivity is a unique and generalized feature in the hypothalamic microcircuit and empathized the prominent roles of neuropeptides in the modulation of microcircuit activity.
This paper entitled “A developmental switch between electrical and neuropeptide communication in the ventromedial hypothalamus” was published online in Current Biology on June 2, 2022. SHAO Yinqi and FAN Liu were the first authors with equal contributions. This work was supported by CAS, NSFC, and Shanghai Government.
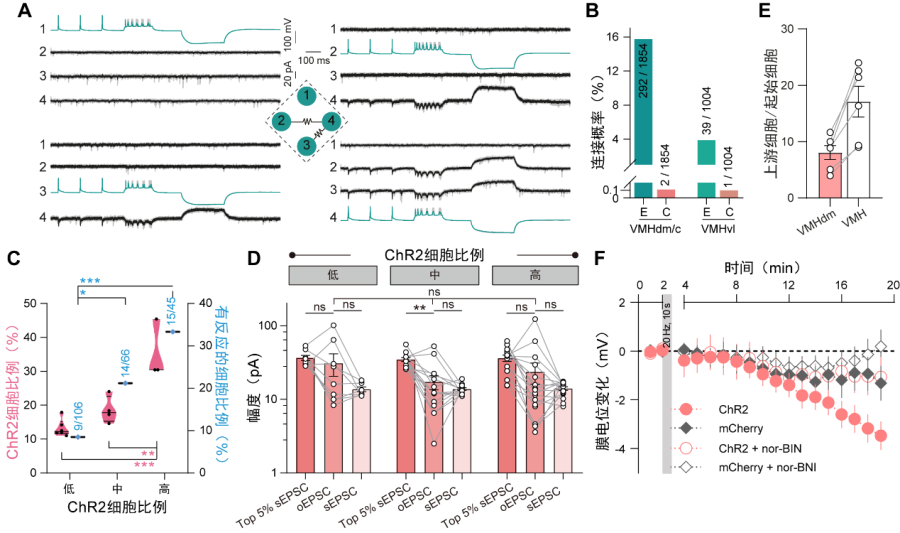
Figure 2 A unique development transition of neural circuit in the ventromedial hypothalamus. (A) Typical traces showing a quadruple whole-cell patch-clamp recording. Electrical synapses were detected between neuronal pairs 2/4 and 3/4. (B) Connection rate of electrical (E) and chemical (C) synapses in the VMH. (C) Fraction of responsive neurons when different proportion (prop.) of VMH neurons were activated. (D) The comparison of peak amplitudes of optogenetic EPSCs (oEPSC) and spontaneous ESPSs (sEPSC). (E) Number of local input cells for each VMH neurons revealed by rabies virus tracing. (F) Membrane potential changes of VMH neurons after high-frequency stimulation and the reversal effect of KOR antagonist nor-BNI.
Keywords: Ventromedial hypothalamus, Neural microcircuit, Paired recording, ChR2-assited circuit mapping, Rabies virus tracing
AUTHOR CONTACT:
XU Huatai
Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.
E-mail: xuhuatai@ion.ac.cn
 附件下载:
附件下载: