Time:2021-09-16
Autism is a neurodevelopmental disease that begins early after birth. Social communication disorder and repetitive, stereotyped interests are the core phenotypes of autism. According to estimates from CDC’s Autism and Developmental Disabilities Monitoring (ADDM) Network, the incidence rate of autism has increased year by year. At present, one in 58 children born is autistic. Attention and research on autism are more and more extensive.
Studies on twins and families have consistently shown that autism has great heritability. For the research on the pathogenic genes of autism, more than 100 risk genes have been found with the continuous progress of sequencing technology. Many gene mutations with clear functions can lead to disease alone. However, among the genetic factors affecting autism, de novo and rare deleterious mutations can only explain 10-20% of autism, while the common genetic and weak functional variants account for a large proportion of autism. SNP is different among regions. Therefore, screening specific SNPs related to autism and studying their functions will provide indispensable evidence for the pathogenic genes and pathogenesis of autism.
QIU Zilong's group and DU Yasong's team collected 167 Chinese autism families, extracted the peripheral blood genome for whole-exome sequencing, screened the risk mutation according to a set of processes, verified the existence of the mutation through Sanger sequencing, and screened a point mutation located in CHD7 (Fig. A).
In order to study whether the point mutation leads to functional defects related to CHD7 deletion, QIU Zilong's group and CHEN Yuejun's group cooperated to construct human stem cells containing CHD7 intronic point mutation by CRISPR / cas9 mediated homologous recombination. During the differentiation of point mutant hESCs into neurons, it was found that the expression of CHD7 decreased, the neural differentiation was delayed, and the morphology of neurons was defective. By comparing the transcriptome of differentiated neurons, they found that the expression level of gene TBR1 increased significantly in differentiated neurons carrying CHD7 intronic variation. TBR1 is an important ASD risk gene. In order to verify whether the change of TBR1 expression level causes the defect of point mutant neurons, the author found that the expression level of TBR1 was up-regulated by knocking down CHD7 in normal cells, which is consistent with the change in point mutant cells. The authors further knocked down the expression of TBR1 in differentiated point mutant neurons and found that the morphological defects of neurons were rescued. The results showed that the defect of point mutant neurons was caused by the change of TBR1 expression level. In order to further study the mechanism of point mutation, the authors analyzed the transcripts of CHD7 and found that besides the normal transcripts, there were three new abnormal forms of transcripts that did not function well (Fig. B).
This work found a genetic point mutation enriched in Chinese autism families and located in the intron of CHD7 gene, verified its function and mechanism on neurodevelopment, provided evidence support for common mutations as the genetic cause of autism, and provided a basis for further understanding the pathogenesis of autism and predicting autism genes.
This work entitled “An Intronic Variant of CHD7 Identified in Autism Patients Interferes with Neuronal Differentiation and Development” was published online in Neuroscience Bulletin on May 4, 2021. ZHANG Ran, HE Hui and YUAN Bo are the first authors with equal contribution. This work was supported by the Chinese Academy of Sciences, Shanghai Municipality, National Natural Science Foundation of China.
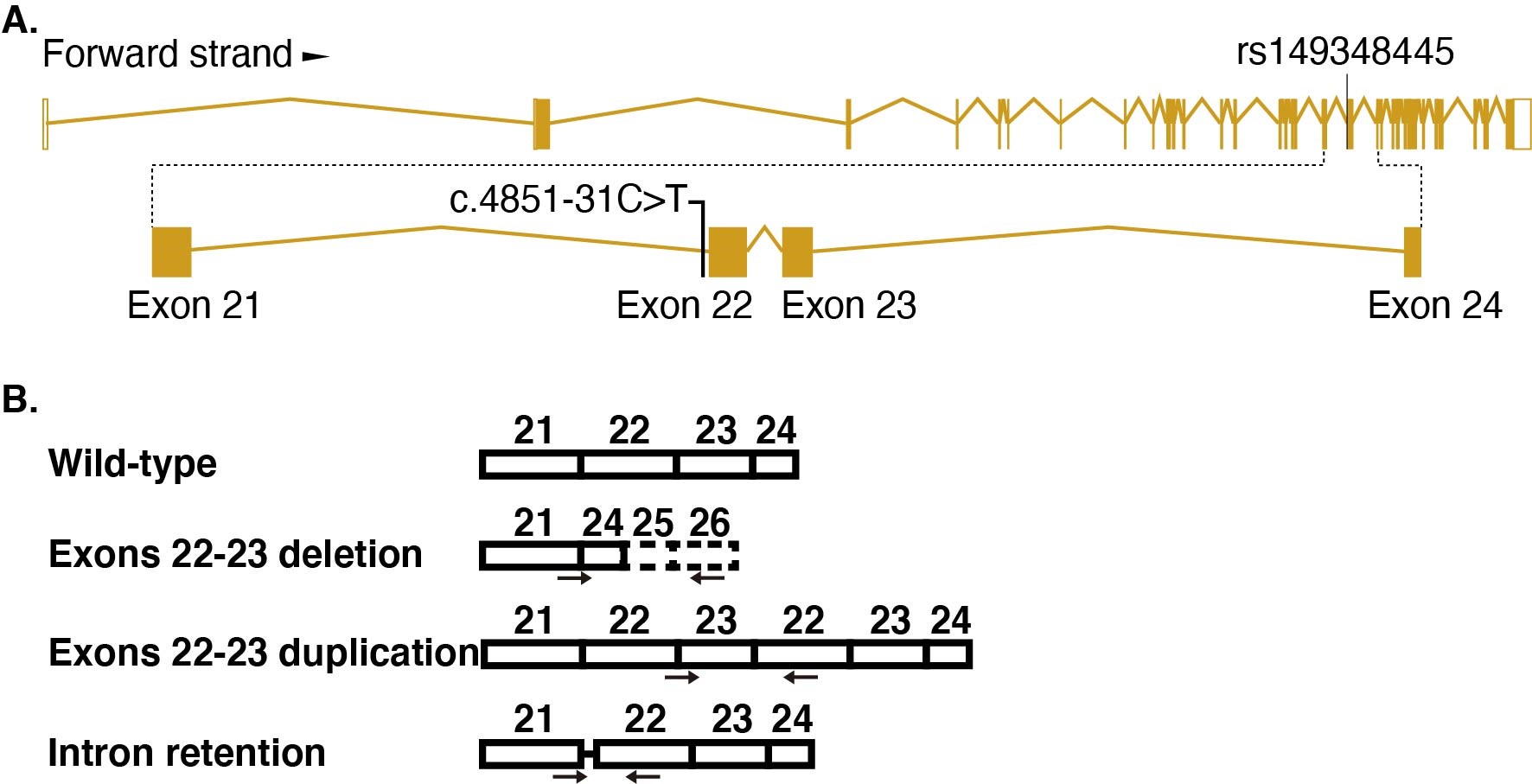
Figure legend: (A) Schematic diagram of point mutation. (B) Diagram of alternative splicing.
http://www.neurosci.cn/content/currentissue/originalarticles_58717/202108/t20210813_657171.html
Keywords: Autism, CHD7, Intronic variant, Inherited variant, TBR1
AUTHOR CONTACT:
QIU Zilong
Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.
E-mail: zqiu@ion.ac.cn
 附件下载:
附件下载: