Time:2021-07-30
Seeing is the process where sensory information from the eyes is processed by our brain to yield subjective perceptions in our consciousness. Visual illusions occur when sensory inputs and perceptual outcomes do not match, and provide a valuable tool to understand transformations from neural to perceptual responses in our brain. A classic example is negative afterimage that remains visible for several seconds after a stimulus is completely removed from view (Figure 1A).
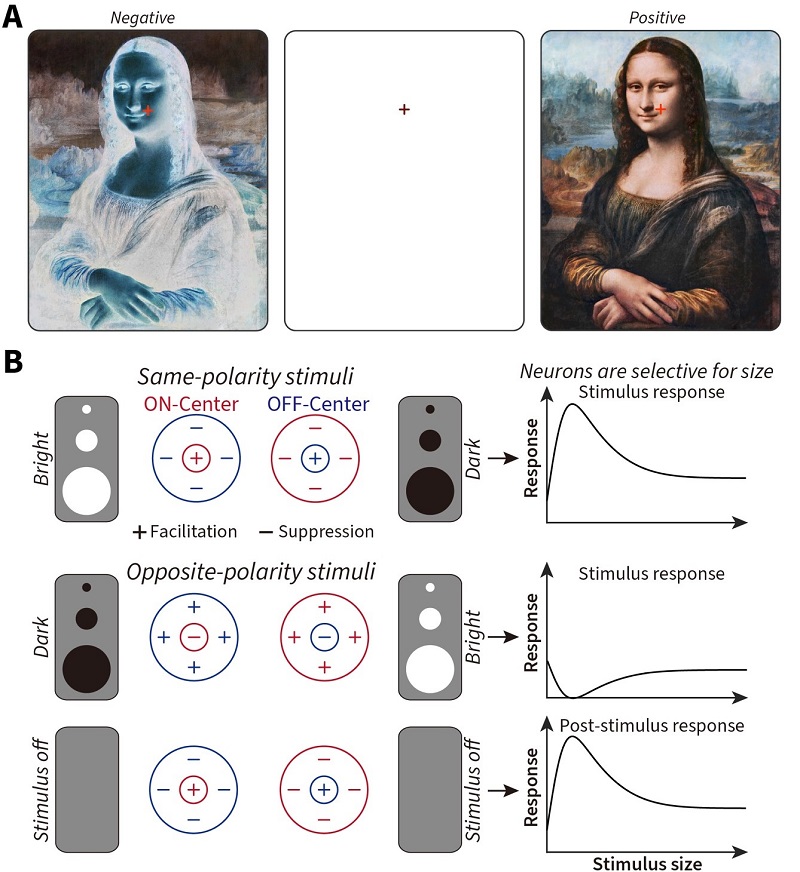
Figure 1 — A: Stare at the red cross over the image on the left for around 30 seconds (the longer you stare, the stronger the illusion), then shift your eyes to the cross on the blank center panel (blinking your eyes helps amplify the strength of the illusory afterimage), and you will see the reversed (positive) painting by Leonardo da Vinci, the Mona Lisa (1503 – 1506 c.e.). Staring at the image on the right for 30 seconds or more and then staring at the center panel will cause you to perceive a negative version of the Mona Lisa. B: The cells in the early visual system share two fundamental features — (1) they respond to bright (ON) and dark (OFF) stimuli differently, exhibiting antagonistic (either + for facilitation or – for suppression) response interactions between their central and surrounding visual regions, and (2) each cell is tuned to different sizes of a stimulus. How do these two fundamental features of visual cells contribute to our perception, especially when the stimulus itself is removed? (Image by CEBSIT)
Usually, dark or bright stimuli evoke inverted bright or dark afterimages, respectively. As such, afterimages have long fascinated philosophers and scientists alike due to this clear difference between physical and perceptual events. Their origin in the brain has been linked to adaptive processes in the transient post-stimulus responses of early visual brain areas. Such areas contain separate channels that respond to bright increments (ON) and dark decrements (OFF) of light selectively. The statistical differences found in natural scenes (more dark decrements than bright increments) have shaped ON– and OFF–channel asymmetries in the brain that are ultimately reflected in differences in our subjective perception of bright and dark negative afterimages (Li et al., 2017). However, the underlying neural mechanisms remain largely unknown.
Combining psychophysics in human subjects, neurophysiological recordings in cats and mathematical modelling, a recent study from Dr. WANG Wei’s lab at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, State Key Laboratory of Neuroscience, reveals how stimulus size and the receptive-field structure of subcortical ON- and OFF-cells contributes to the parallel asymmetries between neural and perceptual responses to bright vs. dark afterimages.
Psychophysically, researchers discovered a size-dependent asymmetry whereby dark disks produce stronger and longer-lasting negative afterimages than bright disks of equal contrast only at sizes above 0.8° of visual angle (a way to measure the size of an object in the visual scene; 1° of visual angle is roughly equivalent to the width of your thumb at arm’s length) (Figure 2A and B). Similarly, neurophysiological recordings from both retinal afferents and relay cells in the feline visual thalamus (dLGN) showed that subcortical ON-cells exhibited stronger sustained post-stimulus responses to dark disks, than OFF-cells to bright disks, at sizes above 1° of visual angle (Figure 2C and D). This was consistent with the psychophysical results (compare Figures 2D to 2A). These sizes agreed with the emergence of differential center-surround antagonism (a core mechanism that visual neurons use when processing visual scenes, see Figure 1B), revealing smaller optimal sizes and stronger surround suppression to opposite-polarity stimuli for OFF-cells (Figure 2E).
Using a network-based firing-rate model of the retina and thalamus (eDOG, extended difference-of-gaussians), the researchers confirmed that stronger surround antagonism, and temporal transience, occurs for OFF-cell post-stimulus rebound responses (Figure 2F). And finally, by using a recurrent spiking neural network model of the area of the brain the dLGN connects to, area V1, they demonstrated that both strength and duration asymmetries can be propagated to downstream cortical areas presumed to ultimately underlie our subjective perceptual outcomes.

Figure 2: A, Tuning curves of the nulling contrast for bright (RED) and dark (BLUE) afterimages across size, along with individual subject scatter plots at each size (grey lines connect the bright and dark points for each subject). B, Mean afterimage duration tuning curves and subject scatterplots for bright and dark afterimages. C Color-map images of response (z-axis) to a series of opposite-polarity stimuli of different diameters (y-axis) at different times (x-axis) after stimuli onset and offset in ON– and OFF–cells, respectively. D, Size tuning of dLGN ON–cells to the offset of dark stimuli and OFF–cells to the offset of bright stimuli. E, ON-cells had a larger optimal size and lower suppression index than ON-cells to opposite-polarity stimuli. F, eDOG model fitted spatiotemporal receptive fields for ON– and OFF–cells. (Image by CEBSIT)
This work reveals the relationship between the receptive field structure and asymmetries between visual ON- and OFF-channels, and provides a deeper link from the underlying neural mechanisms to the resultant perceptual outcomes. It supports the idea that subjective perceptual responses can closely reflect the underlying neuronal receptive field organization of the brain, even when no physical visual stimulus remains in sight.
The study, entitled “From receptive to perceptive fields: Size-dependent asymmetries in both negative afterimages and subcortical ON and OFF post-stimulus responses” was published as a research article online in The Journal of Neuroscience on July 29. This work was mainly conducted by graduate student LIU Xu under the supervision of Dr. WANG Wei and Dr. Ian Max ANDOLINA at the Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences. Collaborators included Dr. WANG Ye of Communication University of China, Dr. WANG Jijun of the Shanghai Jiaotong University School of Medicine, and Dr. Lothar SPILLMANN of the University of Freiburg. This research was supported by the Chinese Academy of Sciences, Shanghai Municipality, National Natural Science Foundation of China and Chinese Ministry of Education.
AUTHOR CONTACT:
WANG Wei, Ian Max ANDOLINA
Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, Shanghai, China.
E-mail: w.wang@ion.ac.cn; i.andolina@ion.ac.cn
 附件下载:
附件下载: