Time:2021-05-11
A recent study published in Nature Communications identified a pathway from the premotor cortex to the superior colliculus that is critical during memory-guided decisions, and dissected the neural circuit mechanisms underlying motor planning. This study was conducted by Xu Ninglong’s lab at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, State Key Laboratory of Neuroscience.
To survive in a dynamically changing world, animals should not always respond to the sensory stimuli reflexively. Instead, they should sometimes hold sensory information in memory, plan an action, and implement the action at an appropriate future time. Imagine a cheetah in the savanna hunting a gazelle. The cheetah needs to hide in the long grass and approaches the gazelle carefully, until it can attack the gazele at the appropriate time. Premature attacks without adequate preparation may scare the prey away, squandering a rare hunting opportunity. Therefore, motor planning is a key process in this life-and-death struggle and in many other adaptive behaviors.
How does the brain plan future actions? Which brain regions and circuits are critical? A previous study showed that the anterior lateral motor cortex (ALM, part of M2) is an important cortical substrate for motor planning (Guo et al., 2014). This work is followed up by several studies that implicated a variety of subcortical brain regions downstream of M2 in motor planning, such as basal ganglia, thalamus, and cerebellum. These interconnected brain regions form a complex motor planning network. But the circuit logic in this network is far from clear. To understand brain-wide circuit mechanisms underlying motor planning, one needs to take a pathway- and cell-type-specific approach. Members in Ninglong Xu’s lab conducted anterograde tracing from M2 and found dense projections in the lateral SC, a midbrain structure previoulsy implicated in planning and executing eye movmenets in primates. The SC also receives basal ganglia input and was shown to be important for voluntary drinking. Based on these anatomical tracing data and past literature, researchers in the Xu lab hypothesized that the pathway from M2 to SC may play a critical role during motor planning.
To test this hypothesis, researchers established a parametric behavior paradigm in head-fixed mice, where mice were trained to discriminate the click rate of sound stimuli, maintain their motor plan during a memory delay, and lick to either left or right lick port to report their choice. Importantly, mice need to wait for a go cue at the end of the delay before they can lick, which forms the motor planning period that gaps sensation and action. Delay lengths and perceptual difficulties were varied across trials, allowing researchers to test the precise role of the circuit during motor planning with different memory demands (figure a-c). Based on results from optogenetic inactivation during different behavior epochs and brain regions, researchers found that both M2 and SC play an important role during motor planning. Simultaneous inactivation of both brain regions caused larger effect than inactivation of M2 alone, indicating that SC does not passively relay M2 information, but actively and additionally contributes to motor planning.
These inactivation experiments inspired the researchers to further ask what kind of task-related information is sent from M2 to SC. To answer that, researchers retrogradely labelled M2 neurons that project to SC and performed in vivo two-photon calcium imaging of these projection-based M2 neurons during mice’s performance. Compared to randomly labeled neurons in M2, cells projecting to SC show stronger choice information coding and this choice information is progressively more selective for the contralateral action as the trial unfolds (figure d-f). These results support that M2-SC circuit carries motor planning information. But are these signals causal for behavior? Researchers conducted chemogenetic manipulation experiments and specifically inactivated the M2-SC circuit without influencing other M2 downstream. M2-SC inactivation selectively impaired mice’s performance on trials with long delays and higher perceptual difficulty (figure g-h). These results support the causal role of M2-SC circuit for motor planning and decision maintenance.
Finally, to test how SC local circuits further process choice information from M2, researchers compared neural activities of SC excitatory and inhibitory neurons during the task. They found that both SC cell types receive M2 input, so they may both further process information from M2 and contribute to motor planning. Using fiber photometry, researchers found that while excitatory neurons encode choice information consistently during sound and delay periods, potentially helping to maintain task information. In comparison, inhibitory neurons showed more dynamic and heterogeneous choice coding, likely playing modulatory roles within the SC (figure i-j). In summary, this study systematically investigated a circuit from premotor cortex to a subcortical collicular region for motor planning and provide insights to the circuit mechanisms underlying motor planning.
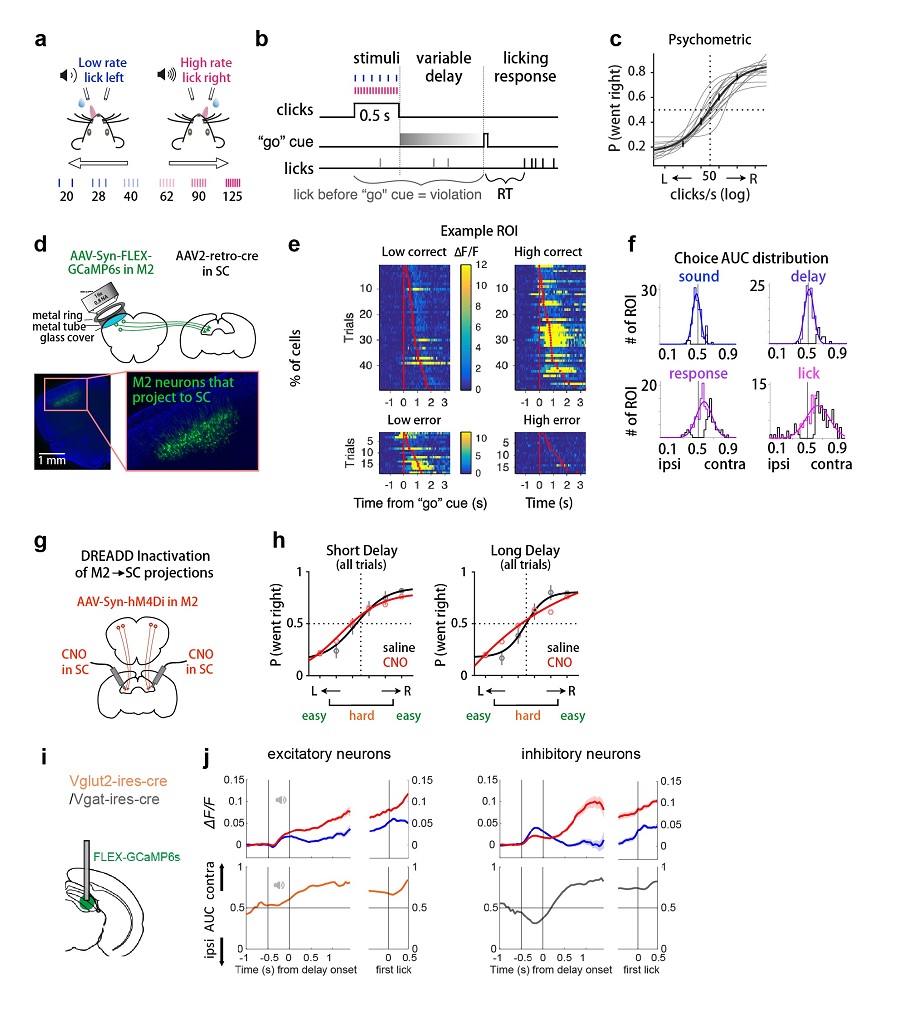
Figure notes:a, auditory two-alternative forced-choice task,mice lick to either of the lick port based on the click rate of the sound stimuli. b, task timing structure. After sensory stimuli, animals need to wait for 0.3-1.5 s until a go cue at the end of the delay before they can lick. c, the psychometric curve of animals. d, two-photon calcium imaging from a subset of M2 neurons that project to SC. e, example neurons showing choice selectivity. f, more and more neurons showing stronger choice selectivity during the trial. g, pathway-specific inactivation of M2-SC circuit using chemogenetics. h, circuit inactivation impaired mice performance in difficult and long delay trials. i, fiber photometry recording of different cell type neurons in SC during motor planning. j, excitatory and inhibitory neurons show distinct activity patterns during motor planning. (Image by CEBSIT)
This work entitled “A cortico-collicular pathway for motor planning in a memory-dependent perceptual decision task” was published in Nature Communications on May 11, 2021. This work was conducted by DUAN Chunyu A. and PAN Yuxin in collaboration, under the guidance of XU Ninglong and help from ZHANG Siyu’s Lab at Shanghai Jiaotong University. In addition, researchers MA Guofen and ZHOU Taotao helped with data collection, and other members of the Xu Lab facilitated the project with active discussions. This study was supported by funding from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, the Chinese Academy of Sciences, and the Shanghai Municipal Science and Technology Major Project.
AUTHOR CONTACT:
XU Ning-long
Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences
E-mail: xunl@ion.ac.cn
 附件下载:
附件下载: