Time:2021-05-06
A recent study published in Neuron unraveled the computational mechanism of a specifically defined neural circuit in the cerebral cortex underlying the rule-based flexible decision-making behavior. This work was performed by the team from Dr. XU Ninglong’s lab at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of Chinese Academy of Sciences, State Key Laboratory of Neuroscience.
To survive and prosper, animals need to make dynamic decisions according to environmental changes. With the evolution of neocortex in mammalian brain, this capability is highly developed in mammals. They not only learn simple stimulus-response associations, but also extract common structures from changing environmental states through latent learning to form internal models, or abstract knowledge. Such prior knowledge effectively guides inference of the hidden environmental states using incomplete information, and thereby facilitate highly adaptive intelligent behaviors. Studying the neural mechanism underlying such flexible decision-making behavior is thus the key to solving the mystery of biological basis of intelligence.
Despite a large number of studies, previous work mainly identified various individual brain regions, including different prefrontal subregions and sensorimotor regions, being related to or involved in behavioral switching upon task rule changes, e.g., Reinert et al, Nature, 2021. But the key question of how these regions interact and coordinate through complex neural circuits to implement computations underlying flexible and intelligent behaviors remains unknown.

To address this question, Dr. XU’s lab combined novel quantitative behavioral methods and cutting-edge neural recording and manipulation technologies to investigate the neural circuit mechanisms underlying inference-based flexible decision-making. They trained laboratory mice to perform a flexible auditory categorization task, wherein the mice showed striking cognitive capability of using prior learned task knowledge to infer hidden task rules and make highly flexible sensorimotor decisions. In each trial, mice were required to categorize sound stimuli as high or low categories depending on the categorization boundary.
In order to test behavioral flexibility, the team introduced two different categorization boundaries that changed in different blocks of trials without explicit cues. After training, mice were not only able to change their choice rapidly after boundary switch, but were also able to use partial feedback information to infer the hidden task state and correctly classify unexperienced stimuli. This intelligent behavior recapitulated the cognitive function of using prior structural knowledge to infer hidden rules and make flexible and adaptive choices, as is often assessed in human subjects using the Wisconsin Card Sorting Test.
To delineate the underlying computational process, researchers in Dr. XU’s lab constructed a reinforcement learning (RL) model incorporating task structural knowledge and state inference. This model exhibited high degree of flexibility while using an efficient prediction error based updating mechanism. This model accurately captured animals’ behavioral performance, and, importantly, quantified trial-by-trial the hidden cognitive variables associated with state inference.
To investigate the neural circuit computation underlying this intelligent behavior, the team performed in vivo two-photo microscopy to image population neuronal activity in the brain during task performance. They found that auditory cortex (ACx) neurons not only represent the sound stimulus information, but also encode a hidden cognitive variable, the estimated categorization boundary, critical for making categorical auditory decisions. This finding represents a rare discovery of how neurons in the brain represent hidden cognitive variables.
To further study how neuronal circuits implement the computational process of updating category boundary estimate based on behavioral feedback, the researchers employed an all-optical recording and manipulation technology to image population activity in auditory cortex while manipulating the top-down projection from orbitofrontal cortex (OFC) to auditory cortex. They found that the top-down OFC-ACx circuit indeed supports the encoding of the hidden variable of auditory categorization boundary. This neural circuit mechanism is consistent with the computational process described by the task state-dependent RL model that the researchers constructed to recapitulate the behavioral results.
Furthermore, to study the causal role of the OFC-ACx circuit in flexible auditory categorization behavior, the team used chemogenetics to silence bilateral projections from orbitofrontal cortex in auditory cortex, and found that only the behavior flexibility but not the basic auditory discrimination was impaired, indicating that the OFC-ACx circuit indeed causally contribute to the flexibility of auditory categorization. This result also corroborates the computational model that the orbitofrontal provided feedback information to update the boundary estimation in auditory cortex.
Finally, to further test this computational model, the team used two-photon microscopy to directly image the activity of OFC-ACx axons, and found that the activity of these axons indeed encode the feedback information important for the boundary estimation updating, as predicted by the computational model.
Taken together, this study employed comprehensive state-of-the-art techniques to obtain measurements at neuronal, circuit- and behavioral levels, and acquired correlational, causal and computational evidence to support a new circuit mechanism in the neocortex implementing the computational process underlying a crucial cognitive function, the rule-based flexible decision-making utilizing task structural knowledge and state inference. This discovery therefore represents an unprecedented advancement in the understanding of neural circuit mechanisms underlying advanced cognitive functions in mammalian brains.
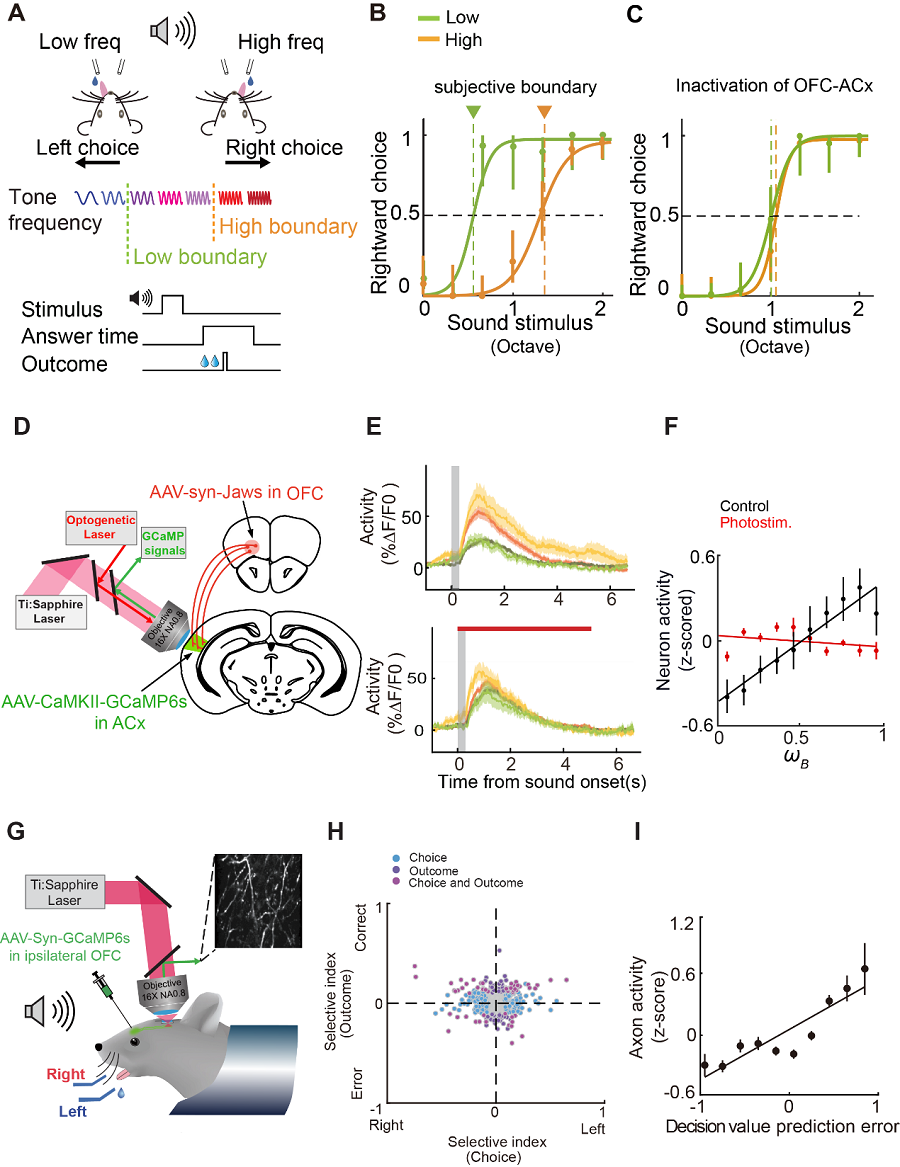
(A) Task design. Mice were trained to report frequency discrimination by licking left and right lick port to obtain water reward. Pure tone stimuli comprise 7 tones equally spaced at logarithmic scale between 7 kHz and 28 kHz. In different blocks of trials, the categorization boundary change was either 10 kHz (low boundary) or 20 kHz (high boundary).(B) Psychometric functions from different blocks of trials with changed category boundary from an example behavior session. (C) Same as (B), psychometric functions from an example session with OFC-ACx projection silenced showing abolished choice switching between different boundary conditions. (D) Schematic showing experimental configuration and optical path design for simultaneous two-photon imaging of ACx neurons using GCaMP6s and optogenetic inactivation of ipsilateral OFC-ACx axons using Jaws during task performance.(E) Calcium signal traces from an example neuron showing selectivity to block type (mean ± s.e.m. across trials). Top, control trials. Bottom, OFC-ACx silencing trials.(F) Activity of block type selective neurons represented boundary estimate of mice. (G) Schematic showing two-photon imaging of OFC axons in auditory cortex during behavior. GCaMP6s was expressed in unilateral OFC using AAV-Syn-GCaMP6s. A chronic imaging window as implanted in ipsilateral ACx to imaging axons projected from OFC.(H) OFC-ACx axons showed selectivity to choice and outcome.(I) OFC-ACx axons revealed feedback information. (Image by CEBSIT)
This work entitled “A cortical circuit mechanism for structural knowledge based flexible sensorimotor decision-making” was published online in Neuron on May 5, 2021. The Ph.D. student LIU Yanhe from Dr. XU Ninglong’s lab was the first author of this study, with experimental and technical help from XIN Yu, CUI Lele and PAN Jinwei in Dr. XU’s lab. This work also benefited greatly from the kind help from the CEBSIT Investigator, Dr. YANG Tianming. This study was supported by grants from National Nature Science Foundation of China, Chinese Academy of Sciences and Shanghai Municipal Science and Technology Commission.
AUTHOR CONTACT:
XU Ninglong
Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.
E-mail: xunl@ion.ac.cn
 附件下载:
附件下载: