Time:2019-04-08
A study published online in Brain on April 1st has revealed a new cellular mechanism for amyotrophic lateral sclerosis (ALS), suggested a novel therapeutic strategy targeting the RNA degradation pathway, and identified an asthma drug as a potential medication for ALS. This work was mainly conducted by researchers from Dr. XU Jin’s lab at the Institute of Neuroscience, State Key Laboratory of Neuroscience, Chinese Academy of Sciences.
ALS is the most common motor neuron disease and one of the most devastating neurodegenerative diseases caused by progressive motor neuron degeneration. There is no cure for the disease and the current treatment options are very limited. Thus, the disease is characterized by fast progression and high lethality. Recent genetic advances have identified a group of new genes, whose mutations contribute to the development of ALS. Among these genes, C9orf72 is the most common genetic cause of familial ALS, and even contributes to sporadic ALS. Unlike commonly seen point mutations and deletions, (GGGGCC)n hexa-nucleotide repeats expansion (HRE) in a non-coding region of C9orf72 is the culprit. Intriguingly, these repeats could generate RNA and protein products and affect RNA metabolism as some other ALS-causing mutant proteins do, although the underlying mechanisms remain to be fully understood.
In this study, by coupling unbiased bioinformatic analysis of various transcriptome studies with validation experiments in multiple C9orf72 cellular and animal models, XU’s team unveiled the inhibition of the nonsense-mediated mRNA decay (NMD) pathway as a conserved consequence of the C9orf72 HRE. NMD is an RNA surveillance machinery vital for the removal of defective or harmful RNA generated from faulty transcription, alternative splicing or viral infection. Key protein components of NMD are found in cytoplasmic structure called processing bodies. Interestingly, XU and colleagues found that HRE-derived neurotoxic dipeptide repeats (DPRs) could inhibit NMD pathway by suppressing processing-body formation while promoting stress granule formation. To test whether NMD pathway could be a potential therapeutic target for ALS, they first genetically reactivated the NMD pathway and found that core NMD genes, such as UPF1, could effectively protect against C9orf72 DPRs neurotoxicity. Next, after evaluating several potential NMD activating compounds, they have identified Tranilast as the most promising NMD-activating drug and found that it could rescue cells and fruit flies from C9orf72 DPRs-induced neurotoxicity. Given that blood-brain barrier-permeable Tranilast has been clinically used to treat asthma with a great safety record since 1980’s, this exciting new study will prompt future pre-clinal and clinal investigations to test the therapeutic potential of Tranilast and other NMD activating compounds in ALS patients with defective RNA metabolism.
This work entitled “Reactivation of nonsense-mediated mRNA decay protects against C9orf72 dipeptide-repeat neurotoxicity” was carried out mainly by Dr. XU Wang-Chao under the supervision of Dr. XU Jin, with contributions from BAO Pu-Hua, Drs. JIANG Xin, WANG Hai-Fang and other members of XU’s laboratory, as well as Dr. Rita Sattler at Barrow Neurological Institute, AZ, USA. This work was supported by National Science Foundation of China grant (81771425). The patent related to this discovery is pending.
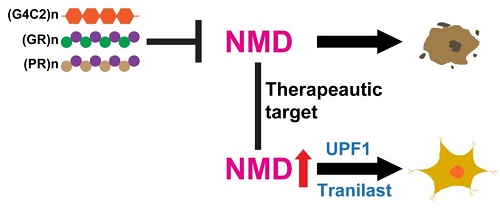
Working model. C9orf72 HREs ((G4C2)n) or arginine-rich DPRs ((GR)n or (PR)n) cause neurodegeneration by inhibiting NMD (top). Conversely, reactivation of NMD by UPF1 expression or Tranilast blocks C9orf72 HRE-mediated neurotoxicity (bottom).
 附件下载:
附件下载: