Time:2018-12-26
A recent study from Dr. ZHU Shujia’s Laboratory etc. published in Cell Reports resolved the first three-dimensional Cryo-EM structure of human GluN1/GluN2A NMDA (N-methyl-D-aspartic acid) receptors at near-atomic resolution. This work demonstrated the structural basis and molecular mechanism of proton inhibition on this key neuronal excitatory receptor.
NMDA receptors belong to the glutamate-gated ion channel families which are widely expressed in the central nervous system (CNS). They play crucial roles in neuronal development and synaptic plasticity. These receptors have always been attracted as research interests in the field of neuropharmacology, as their dysfunctions are involved in a large number of neurological disorders, including stroke, depressive disorder, epilepsy, schizophrenia, Parkinson's disease and Alzheimer's disease. NMDA receptors typically form heteromeric tetramers composed of two copies of glycine-binding GluN1 subunits plus two copies of glutamate-binding GluN2 (2A-2D) subunits. The latter ones mainly determine the biophysical and pharmacological diversity of these channels. The developmental profile of subunit expression in the brain varies among four GluN2 subunits. At early postnatal stage, there is a developmental switch at the synapse from predominantly GluN2B-containing to GluN2A-containing receptors. While previous studies mainly focused on the structure-and-function of GluN1/GluN2B subtype, little structural information is known concerning the GluN1/GluN2A subtype.
During the excitatory glutamatergic neurotransmission, synaptic vesicle exocytosis of the glutamate is always accompanied with proton, which can directly inhibit the channel activity of NMDA receptors. However, the structural basis of proton inhibition on the NMDA receptors is unknown. Jinbao Zhang at Dr. ZHU Shujia’s laboratory screened multiple mutations to stabilize the tetramer and eventually purified the human GluN1/GluN2A NMDA receptor proteins. In collaboration with Dr.CHANG Shenghai at Zhejiang University, they then elucidated the structures of the GluN1/GluN2A receptor under different pH conditions using single-particle Cryo-EM. The researchers found that the N-terminal domain (NTD) of the GluN2A adopts a subunit-unique open and twisted conformation at the high pH. Upon protonation, the GluN2A-NTD domain transits to a closed and untwisted conformation, causing allosteric re-arrangement of NTDs, agonist-binding domains, and gate-associated transmembrane helices (as shown in the cartoon). Meanwhile, this conformational mobility upon protonation is confirmed with in silico molecular dynamics simulations conducted by Pan Xu at Shanghai Institute of Materia Medica. This work enriched the neuropharmacological potentials for the NMDA receptors, and provide important structural information for drug design, screening and development.
The project was mainly conducted by researchers at Dr. ZHU Shujia’s Laboratory at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, in collaboration with the team of Dr. ZHANG Xing at the Center of Cryo Electron Microscopy, Zhejiang University School of Medicine, and the team of Dr. LUO Cheng at Drug Discovery and Design Center, Shanghai Institute of Materia Medica, Chinese Academy of Science. This work was supported by the China State Key Research Grant, the National Natural Science Foundation of China, the Strategic Priority Research Program of Chinese Academy of Science .
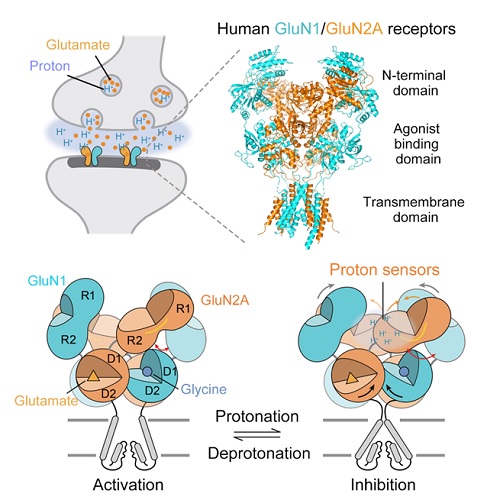
Graphic Abstract: The 3D Cryo-EM structure of human GluN1/GluN2A NMDA receptors. Glutamate and proton are co-released from pre-synaptic vesicle. While glutamate is the primary agonist, protons can inhibit NMDA receptor activity by binding to the N-terminal domains of GluN2A subunits.
 附件下载:
附件下载: