Time:2018-09-28
A recent study published in Neuron demonstrated in vivo that PDGFRβ-expressing mural cells of the brain vasculature function as initial sensors of external insults, and secrete chemokine CCL2 to relay the signal to neurons in multiple brain regions, to increase excitatory synaptic transmission and total neuronal excitability. This study was performed by Dr. YU Xiang’s laboratory at the Institute of Neuroscience, State Key Laboratory of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences. Through their gateway position in the brain, PDGFRβ cells are ideally positioned to respond rapidly to environmental changes and coordinate responses in the central nervous system.
Recent evidence demonstrating extensive crosstalk among the nervous, immune and circulatory systems has sparked much research studying their interactions. Immune cells and molecules have been shown to cross the blood-brain barrier (BBB), under both physiological and pathological conditions. Furthermore, a number of classic immune molecules, including cytokines and cell surface molecules, were found to have important functions in neural development and plasticity. Finally, cells of the nervous and immune systems have been shown to bidirectionally regulate each other.
The response to infection is a physiopathological condition for which crosstalk among the three systems is particularly relevant. Young children are especially vulnerable, as infection is a leading cause of mortality for children under five years of age. If not quickly and effectively treated, systemic infection can lead to central nervous system inflammation, resulting in encephalitis, meningitis, seizures, and sometimes coma. Long-term adverse consequences of neonatal neuroinflammation, partly attributed to excessive immune responses to systemic infection, include intellectual disability, epilepsy, autism spectrum disorder and schizophrenia.
Previous studies have shown that microglia and astrocyte activation, as well as influx of monocytes into the brain parenchyma, together with the cytokines that they release, all contribute significantly to the late phase of infection-induced neuroinflammation. What cells and molecules are key contributors to the early phase of neuroinflammation? In recent work, Lihui Duan and Xiaodi Zhang from the Yu laboratory showed that within 2 hours of induction of systemic inflammation in mice [i.p. injection of lipopolysaccharides (LPS), Poly(I:C) or ODN 1668, to mimic bacterial or viral infection], PDGFRβ mural cells of blood vessels expressed high level of chemokine CCL2. These results were confirmed using three different mouse lines, using immunohistochemistry, combined with in situ hybridization, as well as conditional knockout of Ccl2 from pericytes in Pdgfrb-Cre;Ccl2fl/fl mice. By single cell RNA sequencing, the authors identified Col1a1 and Rgs5 subgroups of PDGFRβ cells as the main source of Ccl2. Both cell types share a number of molecular markers in common with pericytes. These cells also express a number of cytokines, in addition to Ccl2.
What is the function of pericyte-secreted CCL2? Whole cell recordings in acute brain slices showed that CCL2 acts as a neuromodulator to increase excitatory synaptic transmission and neuronal firing in glutamatergic neurons from multiple brain regions, including CA1, CA3 and layer 2/3 pyramidal neurons, as well as dentate granule cells. LPS- or Poly(I:C)-treated medium from human brain vascular pericytes induced similar effects in acute brain slices, in a CCL2-dependent manner. Importantly, when Ccl2 was conditionally knocked out from pericytes in Pdgfrb-Cre;Ccl2fl/fl mice, the effect of LPS-induced increase in excitatory synaptic transmission and in sickness behavior were significantly attenuated. While most experiments were carried out in developing mice, the key findings were reproduced in adult or aging mice, suggesting that they represent a general response to infection. Together, the results provides the first in vivo evidence that pericytes function as the initial sensor of systemic inflammation in the brain, and report, for the first time, that pericytes can regulate the excitability of neurons.
Whether CCL2 or other cytokines secreted by pericytes induce activation of astrocytes and/or microglia during the later phases of neuroinflammation remains to be determined in future studies. Importantly, by virtue of being a sentinel and an early responder to systemic inflammation, pericytes (PDGFRβ, Col1a1 and/or Rgs5 subtypes) are ideal targets for neuroinflammation. Furthermore, their localization at the interface between the circulatory system and the brain parenchyma make them easily accessible to pharmacological agents, without the need for these drugs to cross the blood-brain barrier.
This work entitled “PDGFRβ cells rapidly relay inflammatory signal from the circulatory system to neurons via chemokine CCL2” was published online in Neuron on September 27, 2018. This work was carried out by graduate students DUAN Lihui and ZHANG Xiaodi under the supervision of Dr. YU Xiang, with contributions from MIAO Wanying and other members of the YU laboratory. This work was supported by grants from the National Key R&D Program of China (2016YFA0501000), the National Natural Science Foundation of China (31530030), and the Program of Shanghai Subject Chief Scientist (16XD1404800).
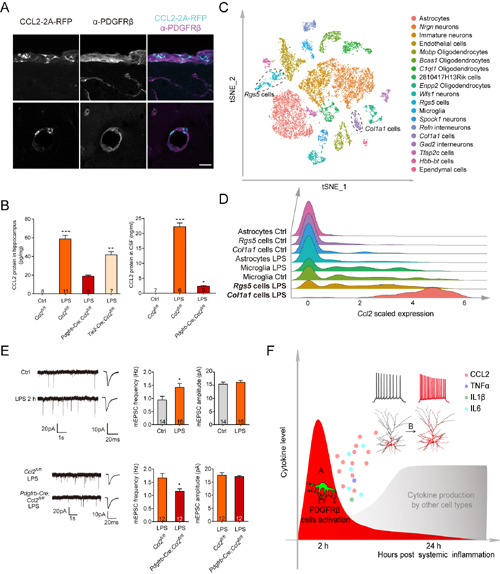
(A) Systemic inflammation (2 hours following LPS injection) induced the expression of Ccl2 in PDGFRβcells.
(B) Pdgfrb-Cre;Ccl2fl/fl mice showed significantly reduced level of CCL2 protein in the hippocampus and cerebral spinal fluid (CSF) 2 hours following induction of systemic infection.
(C, D) Single cell RNA sequencing results showing that Col1a1 and Rgs5 sub-groups of PDGFRβ cells are the main source of CCL2.
(E) LPS treatment increased excitatory synaptic transmission, an effect blocked in Pdgfrb-Cre;Ccl2fl/fl mice.
(F) Working model.
 附件下载:
附件下载: