Time:2017-08-25
A recent study published in Autophagy demonstrated that Mir505-3p regulated axonal development by targeting Atg12 and autophagy pathway. This work was performed by researchers in Dr. ZHOU Yuxun’s team at the Donghua University and Dr. QIU Zilong’s Lab at the Institute of Neuroscience, Chinese Academy of Sciences. This work has successfully constructed a mouse model deleting Mir505 gene based on CRISPR/Cas9 system, and showed that Mir505-3p negatively regulated autophagy to promote axonal development by using in utero electroporation and transmission electron microscopy. This work provided another evidence supporting the role of autophagy in neural development.
“Autophagy”, namely “self-eating”, is a conserved physiological pathway to degrade and recycle senescent organelle and packaged endosome. The vesicle with multiple membranes called “autophagosome” is the most typical structure of autophagy. With packaging and delivery of autophagosome, cargos in endosome have access to lysosome for degradation. Although neurons were applied in observing and identifying ultrastructure of autophagy early in 1970’s, it is still elusive of how this critical pathway participates in regulation of neural development, neural degeneration and regeneration of injured axon. Recently, basic and clinical research on neural autophagy as well as constructing autophagy family genes oriented animal model has become a hot topic in both neural science and medical science.
In a previous study, Dr. ZHOU Yuxun’s team discovered a quantitative trait loci, which controls mouse puberty onset (figure A), and contains Mir505 gene, a potential regulator of this physiological event. Another study showed that the Mir505 located in a quantitative trait loci relative to human X-linked hypopituitarism (figure B). As the essence of puberty onset and hypopituitarism is neural development event, researchers from Donghua University attemped to check whether Mir505 influences neural development by seeking cooperation from Dr. QIU Zilong’s Lab.
The researchers first used cultured cortical neuron model to test whether Mir505 is involved in neural development. They found that the Mir505-3p, one of a mature form of Mir505 gene, specifically promoted polarity establishment, axonal growth and axonal branching with no obvious influence on dendrite. Another mature form of Mir505 gene was not responsible for these phenomena. Researchers observed the similar phenotypes in cortical neuron by adopting in utero electroporation. Next, by integrating results from bioinformatics, RNA sequencing and dual-luciferase assays they screened out a direct target gene of Mir505-3p in neuron, Atg12. Atg12 was a key component of autophagy pathway that encoded ATG12 protein which interacted with ATG5 and ATG16L1, to form a protein complex and to regulate early initiation and elongation of autophagosome. Before this work, it was still unclear whether Atg12 played a role in regulating neural system development.
To investigate the role of Atg12 in regulating neuron development, researchers over-expressed Mir505-3p and Atg12 with lipofection or electroporation in cultured neurons. They found that Atg12 inhibited neuron polarity establishment, axonal growth and axonal branching, which were opposite to the effects of Mir505-3p. Besides, co-expression Mir505-3p rescued Atg12’s effects on axonal development (figure C), indicating the inhibitory role of Atg12 in axonal development. Researchers also observed that Atg12 rescued promotion effects on axonal growth and branching in both the ipsilateral side and the contralateral side of cortical plate caused by Mir505-3p, further demonstrating the inhibitory role of Atg12 on axonal development in vivo (figure D).
To examine whether the Mir505-3p is required for mouse axonal development in vivo, researchers constructed Mir505 gene specific knock-out mice based on CRISPR/Cas9 system. Evidences of HE staining, toluidine blue staining (figure E) and immunofluorescence staining (figure G) showed that the density of axonal bundle was decreased in corpus callosum (cc), cingulum (cg) and fimbria (fi) regions in Mir505 KO mice, comparing to that of WT mice. However, no difference in the cell number of RBFOX3-positive neurons was observed, indicating that attenuation of axons resulted from defects in axonal development, rather than neuronal proliferation (figure G). By utilizing this mouse model, researcher observed obvious alterations of autophagosome in both cultured cortical neuron and mouse cortex tissue (figure F). In Mir505 KO mice, the number and size of autophagosome were drastically decreased, whereas the number of mitochondria was increased. These results showed that deleting Mir505-3p led to general activation of autophagy pathway and Mir505-3p was required for maintaining normal autophagic flow. The axonal development was a high-energy-demanding process, which required mitochondria that were available throughout the neuron, especially the axon, to provide a large amount of ATP. Local distribution and mobility of mitochondria in axons were critical to axonal growth and branching. The observation from transmission electron microscopy revealed that Mir505-3p finally influenced mitochondria number in axon via regulating Atg12/autophagy pathway, which resulted in promotion of axonal development. Finally, researchers showed that Mir505-3p generally regulated autophagy and mitochondria number by targeting Atg12 in primary MEF cells.
In this project, ZHOU’s team and QIU’s Lab established a Mir505 KO mouse model and revealed novel functions of Mir505-3p/Atg12/autophagy/mitochondria pathway on regulating axonal development both in vitro and in vivo, by using cultured cortical neuron, in utero electroporation and transmission electron microscopy (figure H). Taken together, this work provided another evidence supporting the role of autophagy in neural development.
This work entitled “Mir505-3p regulates axonal development via inhibiting the autophagy pathway by targeting Atg12” was published online in Autophagy on August 19th, 2017. Dr. YANG Kan from Donghua University (now the postdoctoral research assistant at the Institute of Neuroscience, Chinese Academy of Sciences) was the first author of this work. YU Bin, CHENG Cheng, CHENG Tianlin and YUAN Bo from the Institute of Neuroscience, Chinese Academy of Sciences were the co-authors of this work. We would like to thank ZHANG Yuefang and SHAN Shifang for their help in animal arrangement and molecular biological supports. We would like to thank HU Qian, XIANG Dan, CHEN Xuxin from the optical imaging facility, and ZHANG Min, QIAN Songlin, JIANG Weifang from the molecular and cellular biology core facility, and HAN Ling from the animal facility, and KONG Yu, PAN Lijun, ZHANG Bin and WANG Xu from the electron microscopy facility, for their technical supports. This work was supported by NSFC Grants (#91432111, #31625013), National Key Scientific Instrument and Equipment Development Program of China (2012YQ03026008), CAS Strategic Priority Research Program (XDB02050400) to QIU, and NSFC Grants (#31371257), the Key Project of Science and Technology Commission of Shanghai Municipality (#14140900502) to ZHOU, CUSF (DH-D-2014049) to YANG.
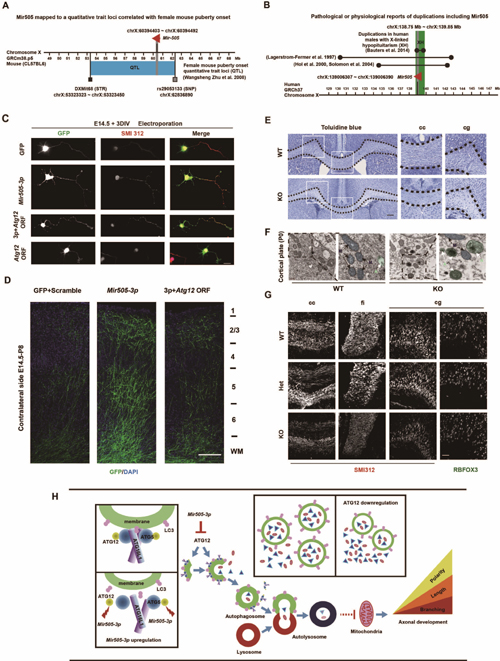
Figure legend: (A) The Mir505 gene is on a quantitative trait loci of regulating female mice puberty onset. (B) Mir505 gene is on a quantitative trait loci of regulating human male X-link hypopituitarism. (C) In cultured cortical mouse neuron, Mir505-3p regulated neural polarity establishment, axonal growth and axonal branching by targeting Atg12 in vitro. (D) Mir505-3p promoted axonal growth and branching of contralateral side of mosue cortex by targeting Atg12. (E) Abnormal morphogenesis of axon bundle in cc region labeled with toluidine staining. (F) Number and area of autophagosome were increased and number of mitochondria was decreased in cortex tissue of Mir505 KO mice with TEM assay. (G) Axonal density was decreased in cc, cg and fi region with IF staining, leaving with no difference of mature neuron number in cg region. (H) Mechanism of Mir505-3p in regulating axonal development via Atg12/autophagy/mitochondria pathway.
 附件下载:
附件下载: