Time:2017-06-22
A recent study entitled “A critical role of pre-synaptic cadherin/catenin/p140Cap complexes in stabilizing spines and functional synapses in the neocortex” from Dr. YU Xiang’s lab at the Institute of Neuroscience, Chinese Academy of Sciences was published in Neuron on June 21, 2017. This study uncovers an asymmetric role for the symmetric cadherin/catenin cell adhesion complexes in functional synapse formation in the neocortex —— pre-synaptic β-catenin is predominant and mediates dendritic spine stabilization through N-cadherin-dependent anterograde trans-synaptic signaling. The effect of the cadherin/catenin requires p140Cap —— a novel β-catenin interacting partner.
Synapses are fundamental building blocks of neural circuits. Synapse formation requires complex regulations involving cell adhesion molecules, secreted molecules, transcription factors and so forth. For cell adhesion molecules, a critical unanswered scientific question is whether the pre- and post-synaptic partners contribute equally to synaptogenesis, or one side is more predominant in inducing functional synapse formation and in stabilizing nascent synapses.
Using Nex-Cre mice to conditionally knockout or overexpress β-catenin in all the neocortical excitatory neurons, and recording from layer 2/3 (L2/3) pyramidal neurons of the mouse barrel cortex, the authors found that β-catenin bi-directionally regulated the frequency of miniature excitatory postsynaptic currents. Furthermore, β-catenin overexpression promoted spinogenesis in L2/3 pyramidal neurons. Using live imaging, the authors showed that β-catenin is required for spine stabilization and not de novo formation. To address the question of whether the pre- or post-synaptic partner is more predominant in inducing or stabilizing nascent synapses, they used distinct transgenic mice and viral injections to specifically manipulate β-catenin in post-synaptic (CaMKCreER and sparse AAV-GFP-β-cat* injection) or pre-synaptic neurons (Scnn1a-Tg3-Cre and dense AAV-GFP-β-cat* injection), and found that β-catenin gain-of-function in pre-synaptic neurons, but not post-synaptic neurons, significantly promoted excitatory synaptic transmission and spine maturation of L2/3 pyramidal neurons.
This surprising result led the authors to test changes in the pre-synaptic loci. Using Ai34D mice which conditionally expressed synaptophysin-tdTomato, a pre-synaptic vesicle-related protein, they found that β-catenin promotes maturation of synaptophysin puncta in pre-synaptic loci. Moreover, β-catenin bi-directionally regulated pre-synaptic release probability of glutamatergic vesicles as measured by electrophysiological recordings. Reported β-catenin binding partners could not mediate the above-described effects. Co-immunoprecipitation and mass spectrometry experiments identified p140Cap, a protein known to regulate exocytosis, as a novel binding partner of β-catenin, and showed the presence of cadherin/catenin/p140Cap complexes. Further experiments demonstrated that pre-synaptic expression of p140Cap is required for excitatory synaptic transmission and spine stabilization.
Together, these results uncover an asymmetric role of the previously thought symmetric cadherin/catenin complex in neocortical circuit wiring. Whether these findings mean generally a more predominant role of the pre-synaptic site in functional synapse formation remains to be determined in future studies. It would also be interesting to explore if this mechanism extends to other neural circuits. The formation and maturation of synapses and spines are fundamental to proper neural circuit wiring and function. A better understanding of the molecular mechanisms underlying these processes is not only important for basic neuroscience, but also of great relevance to understanding of neurodevelopmental disorders with synapse dysfunction, such as autism spectrum disorder.
This work was carried out by graduate student LI Minyin under the supervision of Dr. YU Xiang, with excellent technical assistance from MIAO Wanying, WU Qiuzi and HE Shunji. This work was supported by grants from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB02010000), the Ministry of Science and Technology of China (2016YFA0501000), the National Natural Science Foundation of China (31530030), and the Program of Shanghai Subject Chief Scientist (16XD1404800).
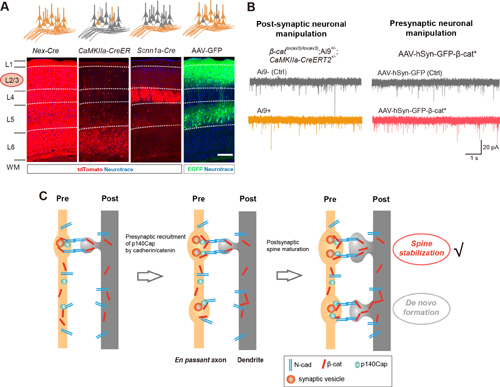
Figure 1:(A) Molecular tools used in the study, including Nex-Cre (expressing Cre in all the excitatory neurons in the neocortex), CaMKCreER (manipulation in post-synaptic neurons), Scnn1a-Tg3-Cre and dense AAV-GFP-β-cat* (manipulation in pre-synaptic neurons).(B) β-catenin gain-of-function in pre-synaptic neurons, but not post-synaptic neurons, significantly increased mEPSC frequency of L2/3 pyramidal neurons.(C) Working model of the role for pre-synaptic cadherin/catenin complexes in post-synaptic spine stabilization.

Figure 2:This image (designed by Dr. Yefei Li) depicts the ornamental braiding (Pan Kou) for fastening the front of traditional Chinese clothing. The fastening is an apparently symmetric structure that is intrinsically asymmetric, as the button needs to pass through the loop on the other side in order to be secured. The image further emphasizes the asymmetry by making the pre-synaptic side (top left) more ornate than the post-synaptic side (bottom right), and depicts the synapse maturation process sequentially (far to near).
 附件下载:
附件下载: