Time:2017-02-06
Recently a research finding describing the critical role of ADAM10-initiated release of Notch intracellular domain in the radial migration of cortical neurons was published online in Cerebral Cortex. In this work, researchers from Dr. XIONG Zhiqi’s lab at the Instititute of Neuroscience, CAS, provided direct evidence showing the function of a new membrane signal paradigm, namely regulated intramembrane proteolysis (RIP), in the regulation of cortical radial migration in mouse brain.
Proper neuron migration is essential to brain morphogenesis and circuit formation, and dysregulation of it usually causes severe brain disorders. Regulated intramembrane proteolysis (RIP), a process in which a transmembrane protein releases its cytoplasmic domain via sequential proteolytic cleavage, has recently been implicated in the regulation of neuronal migration, but the underlying molecular mechanism remains unclear. In this study, the authors used both in utero electroporation and tamoxifen induced-deletion to selectively inactivate ADAM10 in cortical neurons, and found that the radial migration of these neurons was disrupted. Moreover, the function of ADAM10 in migration was found to be dependent on the RIP of Notch as its intracellular domain NICD mitigated the migration defect of ADAM10-deficiency neurons. Furthermore, they found that ADAM10/Notch signaling affected microtubule structure and neuronal motility by regulating the transcription of microtubule-associated proteins (MAPs), which is distinct from formerly identified function of Notch in proliferation and fate control. Specifically, DCX, a critical modulator of microtubule dynamics, was found to be a direct target of the NICD/RBPJ transcriptional activation complex in migrating neurons. Expression of NICD prevented the down-regulation of DCX, and overexpressing DCX significantly restored the level of acetylated tubulin and mitigated the migration defect of ADAM10-deficient neurons.
This work revealed a novel function of ADAM10 during neuronal development, but also demonstrated that ADAM10-mediated RIP functions as a new membrane signal transduction paradigm in the regulation of neuron migration. Together with paradigms such as ligand-receptor binding or interaction between adhesion molecules, ADAM10-initiated RIP of Notch enable newborn neurons with multiple strategies to sense environmental stimuli and precisely orchestrate intracellular molecular machinery for migration. Moreover, accumulating evidence supports of the function of Notch in the regulation of synaptic plasticity, morphogenesis and cell movement, whereas little is known about the underlying molecular mechanism. The discovery that ADAM10/Notch signaling regulates microtubule cytoskeleton dynamics to control neuronal motility provides a general, conceptual advance for how this signaling pathway is involved in these cellular processes. Anti-mitotic or microtubule compounds have been shown with promising therapeutic effect to treat leukemia, which is associated with mutations of Notch1 in human patients. The link between ADAM10-initiated RIP of Notch and microtubule cytoskeleton should help the development of new drugs for the treatment of related diseases. Moreover, the identification of Dcx gene as a direct target of NICD/RBPJ complex provides a specific and direct mechanism underlying the requirement of ADAM10-initiated RIP of Notch in regulating neuronal migration. These findings advanced our knowledge of the molecular mechanisms underlying neuronal migration and brain development.
This research was performed under the supervision of Dr. XIONG Zhiqi, head of the Lab of Neurobiology of Diseases at the Institute of Neuroscience, CAS. Dr. CHENG Xuewen, an associate investigator in the lab, led a team consisting of postdoc YANG Zhi and Dr. CHEN Renchao, LI Pengfei, and work tightly in the design, experimentation, data analysis and manuscript preparation. The animal facility and microscope imaging platform, especially Dr. HU Qian provided critical technical support. This work was supported by grants (81271261,31321091, 91332203) from National Natural Science Foundation of China, grants (XDB02020007, QYZDJ-SSW-SMC010) from Chinese Academyof Sciences and grant (2016YFA0501002) from the Ministry Of Science and Technology.
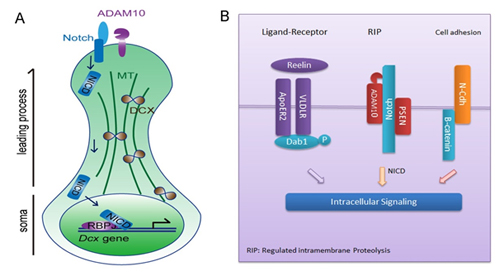
Figure legend A. diagram showing the identified signaling in regulating neuron migration. ADAM10-initiated cleavage of membrane Notch leads to the cytosolic release of NICD, which translocates into the nucleus and forms a complex with RBPJ, thereby promote the transcriptional expression of DCX, which stabilizes microtubule structure and is critical for neuron migration. B. Membrane signal transduction paradigms in the regulation of cortical neuron migration. Together with paradigms such as ligand-receptor binding or interaction between adhesion molecules, ADAM10-initiated RIP of Notch enables newborn neurons with multiple strategies to sense environmental stimuli and precisely orchestrate intracellular molecular machinery for migration.
 附件下载:
附件下载: