Time:2017-02-03
A recent study published in Neuron shows that fibroblast growth factor 13 (FGF13) plays a critical role in the regulation of heat nociception. This research work was carried out by Dr. ZHANG Xu’s Lab at the Institute of Neuroscience, Chinese Academy of Sciences.
Noxious stimuli are of different modalities, including thermal (heat and cold), mechanical and chemical stimuli. Nociception is a type of sensation triggered by noxious stimuli and serves as an important protection to the body. The mechanisms underlying nociceptive modalities remain to be fully explored.
Transduction of nociceptive signals requiresa cascade of molecules with distinct sensory functions. For example, different modalities of nociceptive signals are transferred into electric signals by specific membrane proteins. The electric signals are further transmitted by nociceptive neurons through the activation of a series of ion channels, including sodium channels. Thus far, the most well-studied molecules related to heat nociception are thermosensors, such as the transient receptor potential cation channelV1 (TRPV1). However, accumulated evidence suggests that the discovered thermosensors are partially involved in heat nociception, opening the door for additional players. The present study reveals that FGF13 is critical to the transmission of noxious heat signaling, and that the FGF13 and Nav1.7 interaction during noxious heat stimulation functions as a novel mechanism for heat nociception.
Using the single-cell transcriptome analysis and in vivo electrophysiological recording, ZHANG’s Lab recently has identified 10 types of the dorsal root ganglion (DRG) neurons in adult mice, including 6 types of mechanoheat nociceptors (Li et al., Cell Research, 2016). FGF13 is selectively expressed in the mechanoheat nociceptive neurons (Fig.A). A series of behavioral paradigms were used to assess the nociception of FGF13 conditional knockout mice, with the FGF13 gene specifically deleted in nociceptive neurons. Mice lacking FGF13 in the DRG neurons selectively lost heat nociception, while other sensations were largely unaltered (Fig.B). The fMRI data showed that several brain regions almost lost the signals responding to peripheral noxious heat stimulation (Fig.C). Furthermore, FGF13 interacted with a voltage-gated sodium channel Nav1.7, and increased the Nav1.7current. During the noxious heat stimulation, the FGF13/Nav1.7 interaction was increased to maintain theNav1.7 level in the plasma membrane and therefore sustain the action potential firing, enabling the transmission of nociceptive heat signaling to the central nervous system (Fig.D).The C-terminus of Nav1.7 served as the region mediating the FGF13/Nav1.7 interaction. The effect of interrupting FGF13/Nav1.7 interaction mimicked the phenotype of FGF13 knockout mice. Thus, FGF13 regulates heat nociception by interacting with Nav1.7.
In this research project, ZHANG’s Lab discovered that FGF13 expressed in the mechanoheat nociceptors specifically regulate heat nociception. Moreover, they unveiled the FGF13/Nav1.7 interaction is a critical mechanism for heat nociception. These findings provide a new conceptual advance inpain mechanism and a novel pain therapeutic target.
This research article entitled “FGF13 Selectively Regulates Heat Nociception by Interacting with Nav1.7” has been published online in Neuron on February 2nd, 2017. YANG Liu and DONG Fei are the co-first authors with equal contribution. This work was supported by NNSFC, SPRP (B) of CAS and STCSM.
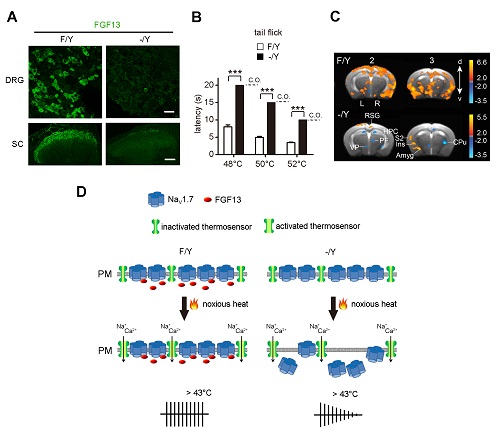
Figure legend: (A) FGF13 is present in nociceptive neurons and their afferent fibers in the dorsal horn of spinal cord of control mice (F/Y). The conditional knockout of FGF13 gene in DRG neurons (-/Y) reduces FGF13 in both the DRG and dorsal spinal cord. (B) FGF13-deficient mice lost the sensitivity to noxious heat stimulation. (C) The fMRI shows the loss of activities induced by noxious heat stimulation in thebrain regions of FGF13-deficient mice.(D) The model ofFGF13 function in heat nociception.During noxious heat stimulation, the FGF13/Nav1.7interaction is increased to maintainthe Nav1.7 level in the plasma membrane and sustain the action potential firing.
 附件下载:
附件下载: