Time:2017-01-06
Researchers from Dr. CAI Shiqing's lab at the Institute of Neuroscience, State Key Laboratory of Neuroscience, Center for Excellence in BrainScience and Intelligence Technology, CAS, demonstrate the critical role of ER-located co-chaperone proteins in regulating potassium channel subunit stabilization and tetrameric assembly.
Ion channels are membrane-spanning proteins that selectively conduct ions across the cell membrane along its electrochemical gradient. Functional ion channels are often formed as assemblies of individual subunits. Protein biogenesis underlying the folding and assembly of ion channels is precisely regulated in order to form normal structure and function of ion channels in the cell membrane. However, the molecular mechanism underlying this process is poorly understood.
The human ether-a-go-go related gene (hERG) encodes a critical K+ channel that controlsrepolarizationof cardiac action potential. Dysfunction of hERG channels results in long QT syndrome (LQTS), a life-threatening cardiac arrhythmia.Through forward genetic screening, the authors found that ER-located chaperone protein DNJ-1 was critical for the biogenesis of UNC-103, aC. elegansERG-type K+ channel. Mammalian DNJ-1 homologues, DNAJB12 and DNAJB14, were also required for the expression of endogenous hERG channels. Knockdown of DNAJB12 and DNAJB14resulted in LQTS-like electrophysiological features in cardiomyocytes: the action potential duration was elongated and early after depolarization appeared. Elevating thechaperone protein level rescued the defective function of hERG mutants found in patients with LQTS. Further studies showed that these chaperones stabilized nascent channel subunits and assembled them into tetramers. The chaperone proteins existed as dimers and tetramers, and oligomerization of chaperones were required for tetramerization of ion channel subunits.
The authors also found that chaperones could promote folding and assembly of a subset of ion channels, suggesting that chaperone-assisted ion channel assembly is a general mechanism. Together, these results show a novel mechanism underlying ER processing of ion channel proteins and suggest that ER-located chaperones may be a potential druggable target for correcting LQTS2 mutation-related protein trafficking abnormalities.
This work entitled " Tetrameric assembly of K+ Channels requires ER-located chaperone proteinsa€? was published in Molecular Cell on Jan. 5th. This study was mainly conducted by graduate student Kai Li and Qiang Jiang under the supervision of Dr. CAI Shiqing. Xue Bai, Mei-yu Ruan and Yi-feng Yang also conducted some experiments. This work was supported by grants from National Natural Science Foundation of China and the Youth Innovation Promotion Association, Chinese Academy of Sciences.
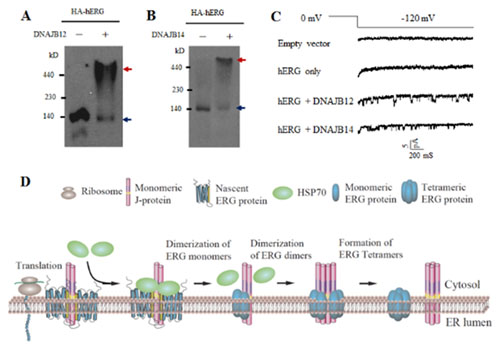
Figure legends: (A) DNAJB12 promote tetramerization of hERG in vitro. (B) DNAJB14 promote tetramerization of hERG in vitro. (C) Single channel currents of hERG channel on co-expression of DNAJB12 or DNAJB14. (D) Proposed model: ER-located chaperone proteins promote tetrameric assembly of ERG ion channels.
 附件下载:
附件下载: