Time:2016-08-10
On Aug 9th, eLife published a paper entitled " The hominoid-specific gene TBC1D3 promotes generation of basal neural progenitors and induces cortical folding in mice" from Dr. LUO Zhenge's group, at the Institute of Neuroscience (ION), Chinese Academy of Sciences (CAS).
It is generally assumed that the expansion of the mammalian neocortex during evolution correlates with the increase in intelligence, and that this process involves increased production of cortical neurons, resulting from an extended neurogenic period, as well as increased proliferative ability of neural stem cells and progenitors. To fit into a limited cranium, expanded cortical surfaces are folded to form gyri and sulci. Recent cross-species studies have shown the emergence of an outer subventricular zone (OSVZ) in the primate cerebral cortex, consisting of a massive pool of proliferating basal progenitors (BPs) and post-mitotic neurons. However, the causal relationship between BP generation and cortical folding still needs experimental confirmation.
Gene duplication may play critical roles in brain evolution. In particular, duplication of specific genes in humans may be responsible for the marked increase in cortical folding. The hominoid-specific gene TBC1D3 undergoes segmental duplications during hominoid evolution, but it's role in brain development has not been explored. In this work, researchers of Dr. LUO's team used in utero electroporation to expression TBC1D3 in ventricular cortical progenitors of mice, and found that it caused delamination of ventricular radial glia cells (vRGs) and promoted generation of self-renewing basal progenitors with typical morphology of outer radial glia (oRG), a cell type most abundant in primates. Furthermore, down-regulation of TBC1D3 in cultured human brain slices decreased generation of oRGs. Interestingly, localized oRG proliferation, resulting from either in utero electroporation or transgenic expression of TBC1D3, was often found to underlie cortical regions exhibiting folding. Together, these results identified a hominoid gene that is required for oRG generation during the regulation of cortical expansion and folding. The transgenic mice generated in this study may provide a feasible model to link cortical folding to higher brain functions.
This study was mainly conducted by graduate students JU Xiangchun and HOU Qiongqiong under the supervision of Dr. LUO Zhenge, with the help from colleagues of several institutions within and outside of CAS, and supported by grants from National Key Basic Research Program of China, National Natural Science Foundation of China, and the Strategic Priority Research Program of the Chinese Academy of Sciences.
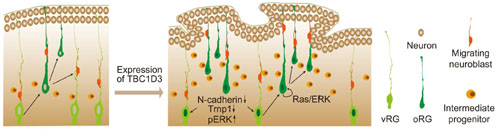
Proposed model for the role of TBC1D3 in cortical folding. TBC1D3 expression causes delamination of vRG cells, through down-regulating the level of N-cadherin and Trnp1, and promotes proliferation of oRG-like cells by regulating cell stemness pathways, including Ras/ERK signaling. The increased generation of the oRG-like cells, the IP cells, and subsequently regional increase in the density of new born neurons, induces cortical folding in mice.
 附件下载:
附件下载: