Time:2015-11-20
Alzheimer’s disease (AD) is the most common neurodegenerative disease leading to dementia in the aged. Over-production and accumulation of β-Amyloid (Aβ) peptide is critical for the pathogenesis of AD. Generation of Aβ involves cleavage of amyloid precursor protein (APP) by γ-secretase, a protease known to cleave several substrates, including Notch. Attempts to treat AD patients by inhibiting γ-secretase activity have been disappointing. Finding specific modulators for γ-secretase could be another avenue to treat the disease.
In a recent study, researchers at the Institute of Neuroscience, Chinese Academy of Sciences, reported that Transient receptor potential canonical (TRPC) 6 blocked the interaction between APP and γ-secretase to specifically suppress Aβ production. TRPC6 specifically interacts with APP (C99), but not with Notch, and prevents C99 interaction with presenilin 1 (PS1), leading to inhibition of γ-secretase cleavage of APP and Aβ production. A fusion peptide derived from TRPC6 also reduced Aβ levels without affecting Notch cleavage.
These results demonstrated that preventing APP interaction with PS1 via TRPC6 could be a novel strategy to reduce Aβ formation. Finding molecule(s) with similar function to TRPC6 or its peptide derivative may provide novel intervention candidates to treat AD patients, while circumventing the side effects induced by direct inhibition of γ-secretase activity.
The paper entitled“TRPC6 specifically interacts with APP to inhibit its cleavage by γ-secretase and reduce Aβ production”was published online in Nature Communications on 19th November 2015. Dr. WANG Junfeng, LU Rui from the Institute of Neuroscience, CAS, and Dr. YANG Jian from Capital Medical University were the first authors, Dr. WANG Yizheng and Dr. WANG Xiaomin were the corresponding authors.
This work was supported by the 973 Program from the Ministry of Science and Technology of China and the grants from National Natural Science Foundation of China.
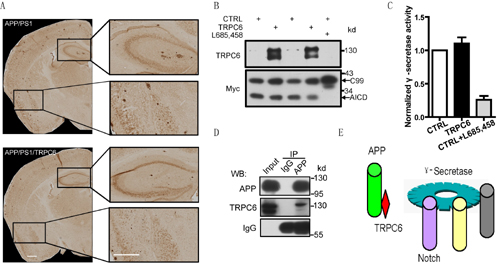
A, The plaque load was significantly decreased in APP/PS1/TRPC6 mice compared with APP/PS1 mice. B, TRPC6 inhibited AICD production from γ-secretase cleavage of C99. C, TRPC6 did not affect γ-secretase activity. D, APP and TRPC6 were in the same complex in the co-immunoprecipitation experiment. E, The model: TRPC6 specifically interacted with APP, inhibited its cleavage by γ-secretase, without effect on γ-secretase cleavage of other substrates, like Notch.
 附件下载:
附件下载: