Time:2015-08-07
In a recent study published online in Cell, entitled “Coordinated spine pruning and maturation mediated by inter-spine competition for cadherin/catenin complexes ”, Dr. YU Xiang’s research group at the Institute of Neuroscience, Chinese Academy of Sciences, demonstrated a novel mechanism through which the pruning and maturation of dendritic spines in the developing brain are coordinated by the cadherin/catenin cell adhesion complex.
Our brain consists of approximately 100 billion neurons, interconnected through synapses to form an enormous network and responsible for our sensation, motion, memory and emotion. Most excitatory synapses in this network are located on structures known as dendritic spines. The number of dendritic spines rapidly increases in early life, accompanying the massive formation of connections between neurons. Interestingly, during the transition through adolescence, spine numbers decrease significantly, in a process where some existing connections are pruned or eliminated to optimize the overall circuitry. This spine pruning process is believed to play critical roles in normal brain function, and its defects have been implicated in developmental neurological disorders such as autism spectrum disorder and schizophrenia. Nevertheless, the molecular mechanism mediating this fascinating process is still poorly understood.
To investigate the molecular mechanism underlying spine pruning, Dr. YU’s group first characterized the spine pruning process in the barrel field of mouse somatosensory cortex (S1BF), which receives sensory inputs from the whiskers. They found that spine pruning in this region was coordinated with spine maturation and bidirectionally regulated by sensory experience, i.e. both processes were accelerated by enriched sensory experience and blocked by whisker deprivation. They hypothesized based on these observations that the pruning and maturation of different spines are the outcomes of the same competition process: neighboring spines compete for certain limited molecular resources, with the winner surviving and maturing, and the loser being pruned.
To identify this limited molecular resource, Dr. YU’s group performed live imaging experiments in cultured hippocampal neurons and found that the locally enriching for a group of cell adhesion molecules, namely the cadherin/catenin complex, simultaneously induced the enlargement of the manipulated spine and shrinkage/elimination of a neighboring spine. Similar spine fate differentiation can also be induced by activating a spine but not its neighbor optogenetically, leading to enlargement of the active one at the expense of its neighbor. This fate differentiation depends on the redistribution of cadherin/catenin complexes between the two spines, inter-spine distance and N-cadherin motility, but not on protein synthesis or degradation, further supporting the competition-based model.
To verify the competition-based model in vivo, Dr. YU’s group combined mouse genetics with viral tools to selectively increase cadherin/catenin-dependent adhesion in a subset of spines in S1BF at the onset of pruning. They found that the manipulated spines survived the pruning process with significantly higher probability while their neighboring spines were more likely to be pruned. Further experiments demonstrated the regulation of spine pruning and maturation by neural activity also relies on the cadherin/catenin complex.
Together, these results identified inter-spine competition for the cadherin/catenin cell adhesion complex as a novel mediator of coordinated spine maturation and pruning. The competition-based mechanism of spine pruning uncovered in this work expands our knowledge of the molecular mechanism mediating neural circuit refinement, and further reinforces the importance of natural sensory stimulation to neural circuit development and plasticity. Furthermore, redistribution of a limited resource to “more needed locations” may represent a general optimization strategy employed by biological systems. Lastly, but importantly, given that defects in spine pruning have been implicated in developmental neurological disorders including autism and schizophrenia, better knowledge of the molecular mechanism mediating spine pruning would contribute to our understanding of these disorders
This work was carried out by graduate student BIAN Wenjie under the supervision of Dr. YU Xiang, with active participation by all members of the laboratory. The study was completed at the Institute of Neuroscience and State Key Laboratory of Neuroscience, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, and supported by grants from the Ministry of Science and Technology and the National Natural Science Foundation of China.
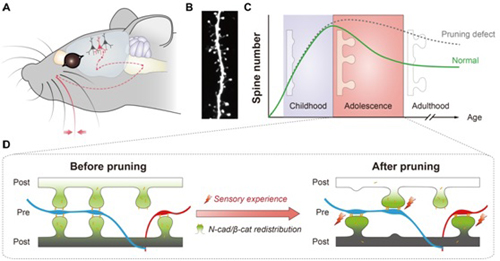
Figure (A) The mouse whisker receives sensory stimuli and send the information to neurons in the somatosensory cortex. (B) An example image showing spines on the basal dendrites of layer 2/3 pyramidal neurons in the barrel field of mouse somatosensory cortex. (C) Developmental dynamics of the spine number of neurons in somatosensory cortex under normal or pruning defect conditions. (D) A competition-based model for cadherin/catenin-mediated spine pruning. The increase of neural activity induced by sensory experience leads to the inter-spine competition for intracellular cadherin/catenin complexes, which results into the redistribution of these complexes in individual spines. The winner spine getting more cadherin/catenin complexes matures, while the loser spine losing the complexes is destabilized and pruned.
 附件下载:
附件下载: