Time:2012-12-20
Neuroglial cells, including microglia and astrocytes, are essential for the maintenance of brain homeostasis. Activated neuroglial cells contribute to immune deregulation and neuroinflammation, which are associated with aging and a variety of neurodegenerative disorders. However, the molecular and cellular mechanisms underlying the regulation of innate immunity in the central nervous system remain elusive.
A team led by Dr. ZHOU Jia-wei, at the Institute of Neuroscience and State Key Laboratory of Neuroscience, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, identified astrocytic dopamine D2 receptor (Drd2) as an important component of the neural network controlling innate immunity in the brain. They showed that astrocytic Drd2 tightly controls the expression of anti-inflammatory protein αB-crystallin (Cryab), which accounts for the observed modulation of immune balance. By influencing Cryab expression, Drd2 modulates inflammatory response and maintains balance of the immune state, representing a completely novel function in the brain. Microglia has long been considered as a key player in neuroinflammation. However, these new results suggest that astrocytes likely play a previously unexpected but critical role in the modulation of neuroinflammation in the context of Drd2 deficiency. These findings open up new avenues in the investigation of brain aging and neuroinflammation-associated brain disorders.
This work entitled “Suppression of neuroinflammation by astrocytic dopamine D2 receptors via αB-crystallin” was published online in Nature on December 16, 2012. This study was carried out by graduate students SHAO Wei, ZHANG Shu-zhen and their co-workers TANG Mi, ZHANG Xin-hua, ZHOU Zheng and YIN Yan-qing from the laboratory of Dr. ZHOU Jia-wei, in collaboration with other Chinese and foreign scientists. This project is sponsored by the Chinese Academy of Sciences, the Chinese Ministry of Science and Technology, and the Shanghai Municipal Government.
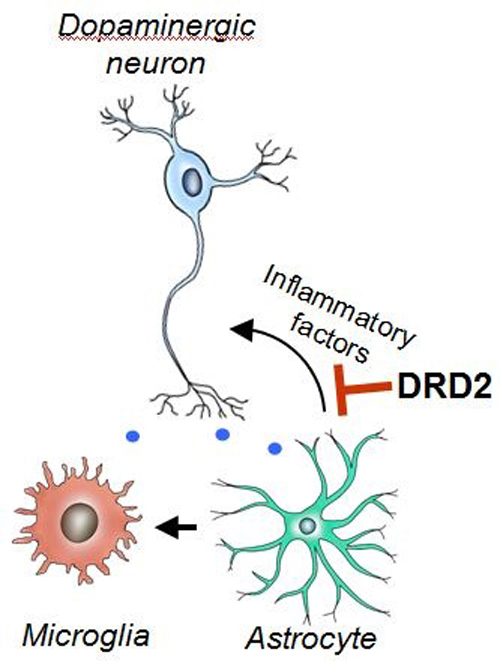
Astrocytic dopamine D2 receptor (Drd2) functions to suppress neuroinflammation by inhibiting pro-inflammatory gene expression via αB-crystallin.
 附件下载:
附件下载: