Time:2010-10-08
On October 7th, a research article entitled “A Nonproton Ligand Sensor in the Acid-Sensing Ion Channel” was published in Neuron. This work was carried out by postdoctoral fellow Dr. Ye Yu, graduate student Wei-Guang Li and co-workers from Dr. Tian-Le Xu’s laboratory at ION, in close collaboration with graduate student Zhi Chen and collaborators from Dr. Hua-Liang Jiang’s laboratory at the Shanghai Institute of Materia Medica.
Acid-sensing ion channels (ASICs) have long been considered as extracellular proton (H+)-gated cation channels and are involved in sensory perception and integration. We previously demonstrated that ASICs are important mediators for ischemic cell death and chronic pain, presumably due to the tissue acidosis. Here, we report the identification of a nonproton ligand sensor in the AISC3 channel. We first showed that 2-guanidine-4-methylquinazoline (GMQ) can induce persistent activation of ASIC3 channel at normal pH. Using GMQ as a probe and combining mutagenesis with covalent modification analysis, we then uncovered a novel ligand sensor in the extracellular ‘palm’ domain of the ASIC3 channel. Moreover, GMQ can activate sensory neurons and cause pain-related behaviors in an ASIC3-dependent manner, suggesting functional significance of ASIC activation by nonproton ligands. Thus, we surmise that natural ligands beyond protons may activate ASICs under physiological and pathological conditions through the nonproton ligand sensor, serving for channel activation independent of abrupt and marked acidosis. These findings are discussed in a Preview by Bagriantsev and Minor (http://mail.cell-press.com/go.asp?/bECE001/qMS3YN1F/xMBWPN1F, see the following figure and its legend), and 'Spotlight On' at the Neuron website.
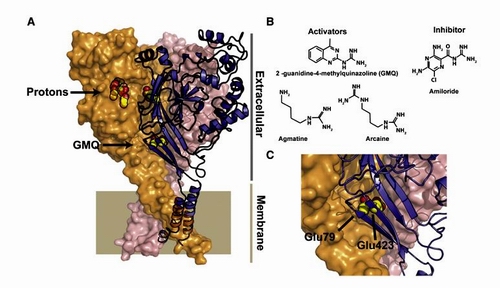
ASIC Structure, Modulators, and Sites of Action
(A) Structure of ASIC1 (3HGC) (Gonzales et al., 2009). Conserved acidic pairs in the putative proton sensing pocket and those that affect GMQ action are shown in yellow and red. Putative proton sensing pocket and the site of GMQ covalent modification are shown. Extracellular and membrane-spanning regions are indicated. (B) Chemical structures of GMQ, agmatine, arcaine, and amiloride. (C) Close-up of the positions of the conserved Glu79 and Glu423 residues. The structure is from ASIC1. The labels correspond to the ASIC3 numbering (adapted from Bagriantsev and Minor. Neuron, 2010, 68: 1-3).
This study was supported by grants from the National Natural Science Foundation of China, the National Basic Research Program of China, and the Shanghai Municipal Government. Dr. Ye Yu is a postdoctoral fellow supported by the K. C. Wong Education Foundation (Hong Kong) and the China Postdoctoral Science Foundation.
 附件下载:
附件下载: