Time:2023-04-11
By AMBYMITCH LESLIE | science.org | Updated: 2023-04-06 11:00
Scientists hope these "pseudoembryos" will boost research into early development, miscarriages
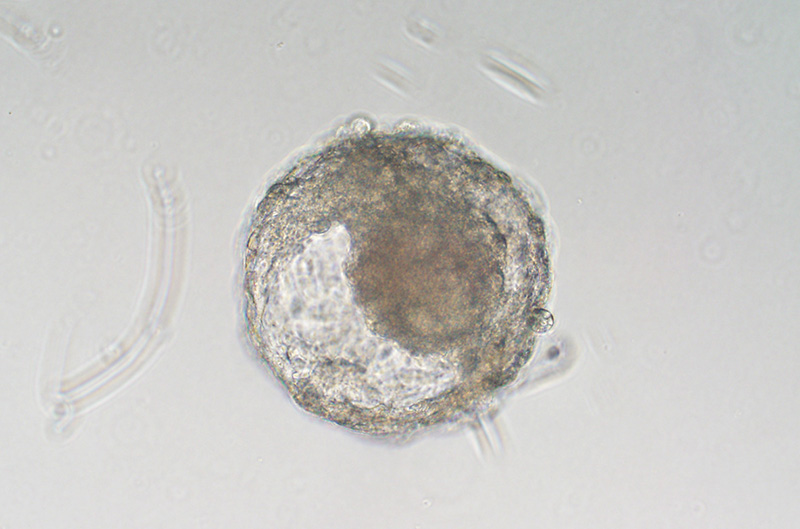
embryolike ball of cells, known as a blastoid, grown from monkey embryonic stem cell
Researchers in China grew this embryolike ball of cells, known as a blastoid, from monkey embryonic stem cells. The researchers were the first to produce monkey blastoids, which could serve as good models of early human development.ZHEN LIU
To probe the mysterious early stages of development, researchers have concocted a variety of embryo stand-ins from mouse or human stem cells. Now, scientists in China have created the first monkey versions. These pseudoembryos should more closely reflect human development than their mouse equivalents. And unlike human embryo mimics, they can be inserted into females to help scientists better understand the beginnings of pregnancy—and why it often fails.
“The findings are a milestone in the field of stem cell–derived embryo models,” says stem cell biologist Alejandro De Los Angeles of the University of Oxford, who wasn’t connected to the study.
Starting with stem cells obtained from embryos or from adult cells transformed into an embryonic-like state, several groups of researchers have grown structures that resemble the blastocyst, the ball of cells that in humans takes shape about 5 days after fertilization and implants in the uterus. Termed blastoids, these imitation embryos can survive for several days in culture and develop many features of the real things. Scientists have even inserted mouse blastoids into mother rodents and shown that they induce some of the changes of pregnancy, although they don’t continue to develop. But mouse blastoids can only reveal so much about human development, and it would be unethical to implant human blastoids into people.
Monkey blastoids promise to be better models, but the right recipe for culturing them proved elusive. Now, Zhen Liu of the Chinese Academy of Sciences and colleagues have generated blastoids from the embryonic stem cells of cynomolgus monkeys, they report today in Cell Stem Cell. The researchers raised the cells in 3D cultures and coaxed them to divide and specialize with two kinds of media. In culture, the blastoids could survive about 18 days.
They developed further than any previous blastoids, undergoing gastrulation, the cellular reorganization that sets up the three basic layers of the embryo. They also contained many of the same types of cells genuine monkey blastocysts do and showed similar patterns of gene activity, suggesting they were faithful replicas.
In previous blastoids, certain types of “founder” cells that give rise to key embryonic structures were scarce, notes developmental biologist Jennifer Nichols of the University of Edinburgh. But the new culture system produced a better balance of these cells, suggesting it might benefit other labs trying to nurture blastoids, she says. A next step, De Los Angeles says, is to investigate ways to prolong the development of the monkey blastoids.
The researchers also inserted 7-day-old blastoids into eight mother monkeys. In three of them, hormones that signal pregnancy appeared in the blood. These three monkeys also sprouted gestational sacs, telltale structures in the uterus that indicate pregnancy. Those findings suggest that monkey blastoids can implant into the uterus and emulate aspects of pregnancy.
They did not continue to develop inside the surrogate mothers, however, suggesting they are not perfect copies. To Nichols, “It’s reassuring that they didn’t develop further.” She thinks that failure should discourage disreputable individuals from trying to pitch human blastoids as a fertility treatment.
“This is a beautiful study,” says Nicolas Rivron, a stem cell biologist at the Austrian Academy of Sciences’s Institute of Molecular Biotechnology whose team engineered the first mouse blastoids in 2018. Creating simulated embryos for more species, he says, will help scientists learn more about early development and what’s necessary for successful pregnancy.
In particular, Rivron hopes the monkey blastoids can provide insights into why implantation often fails in humans. “Implantation is the bottleneck of human pregnancy,” he says.
 附件下载:
附件下载: