Time:2020-04-06
A recent study analyzed the genomic, behavioral, multi-channel EEG and functional MRI data of transgenic macaque monkeys, and found that MECP2 gene overexpression caused a chain of functional changes in GABA signaling pathway, β band neural synchronization, functional connectivity networks and cognitive behaviors. This work was performed by researchers in Dr. WANG Zheng’s lab at the Center for Excellence in Brain Science and Intelligence Technology, Institute of Neuroscience, State Key Laboratory of Neuroscience, Chinese Academy of Sciences. Furthermore, it identified that abnormalities of functional connectivity networks in transgenic macaques were similar to those of a subgroup of autism patients.
Autism spectrum disorder (ASD) is a complex disorder with co-occurring symptoms caused by multiple genetic variations and brain circuit abnormalities. To dissect the gene-circuit-behavior causal chain underlying ASD, researchers used genetically-engineered macaque monkeys with extra copies of MECP2 gene, which has been demonstrated casual effects in humans, to examine how MECP2 overexpression causes influence on gene co-expression, brain circuits and behaviors, and how these deficits correspond to human autism. They first conducted a whole-genome expression analysis, which revealed that MECP2 co-expressed genes were significantly enriched in GABA-related signaling pathways (Panel A). Next, autism-like behaviors including stereotypic locomotion and cognitive inflexibility were evaluated in two behavioral paradigms (Panel B and C). Brain network dysfunction underlying autism-like behaviors were then explored using multi-channel EEG recording and resting-state fMRI. They found reduced beta synchronization within fronto-parieto-occipital networks in transgenic monkeys, which was associated with abnormal locomotive behaviors (Panel D). Meanwhile, they found hyper-connectivity in prefrontal and cingulate networks, which accounted for regressive errors in reversal learning tasks (Panel E). Furthermore, they stratified a cohort of 49 autisms and 72 controls out of 1112 subjects using functional connectivity patterns, and identified dysconnectivity profiles similarly observed in monkeys (Panel F). For the first time, the authors demonstrate that the circuit abnormalities linked to MECP2 and autism-like traits in the monkeys can be mapped to a homogeneous ASD subgroup, thereby offering a new strategy to deconstruct clinical heterogeneity in ASD.
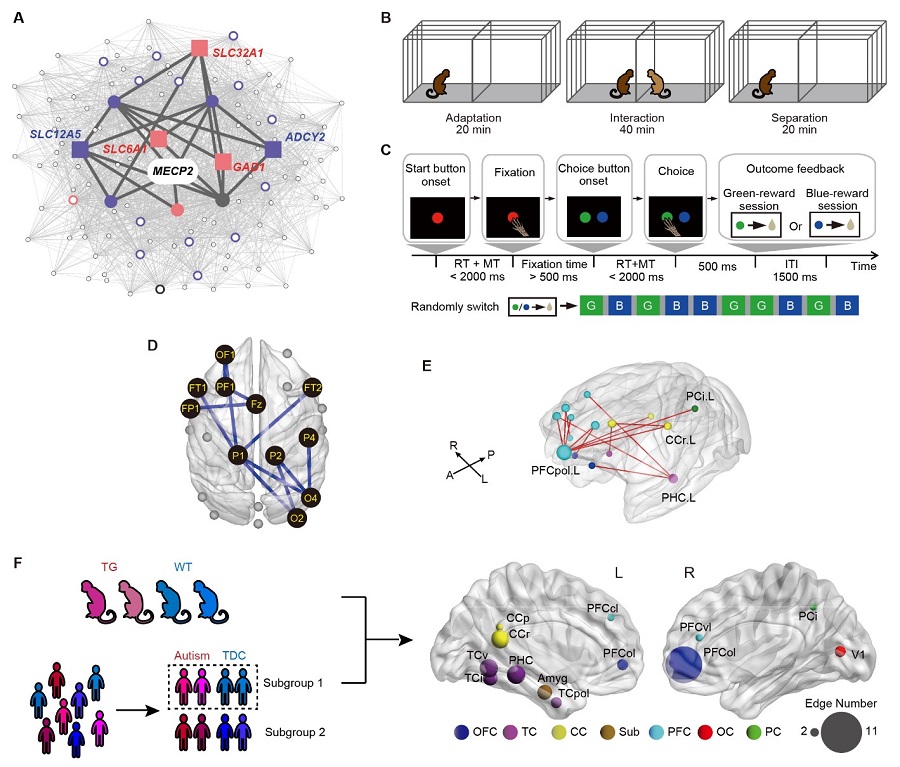
Figure legend: (A) MECP2 co-expression network with five genes enriched in GABA-related pathways. (B) Peer separation paradigm for evaluation of locomotive impairment. (C) Reversal learning task for evaluation of cognitive flexibility. (D) Reduced beta synchronization within fronto-parieto-occipital networks in transgenic monkeys. (E) Increased functional connectivity within prefrontal and cingulate networks in transgenic monkeys. (F) Abnormal brain regions shared between transgenic monkeys and a subgroup of human autistic patients.
This work entitled "MECP2 duplication causes aberrant GABA pathways, circuits and behaviors in transgenic monkeys: neural mappings to patients with autism” was published online in the Journal of Neuroscience on April 8, 2020. This work was completed by Dr. CAI Danchao, Dr. WANG Zhiwei, Ms. BO Tingting and Ms. YAN Shengyao, under the supervision of Profs. WANG Zheng and Ge Shengjin. This work was supported by the National Key R&D Program of China, the Strategic Priority Research Program of Chinese Academy of Science, grants from National Natural Science Foundation, Shanghai Municipal Science and Technology Major Project, and Key Realm R&D Program of Guangdong Province.
AUTHOR CONTACT:
WANG Zheng
Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.
E-mail: zheng.wang@ion.ac.cn
 附件下载:
附件下载: